
The authors describe the factors affecting reconstitution time of dry powder for injection and classifies them as intrinsic and extrinsic parameters.
Arvind K. Bansal is an associate professor at the National Institute of Pharmaceutical Education and Research, Sector-67, Phase-X, S.A.S. Nagar, Punjab 160062, India, tel 191 0 172 2214682 87, fax 191 0 172 2214692.

The authors describe the factors affecting reconstitution time of dry powder for injection and classifies them as intrinsic and extrinsic parameters.

The selection of an appropriate salt form for a potential drug candidate is an opportunity to modulate its characteristics to improve bioavailability, stability, manufacturability, and patient compliance.
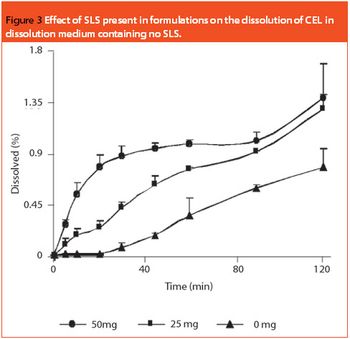
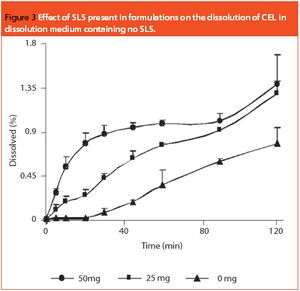
Bioequivalence with the reference product is the only reliable measure of demonstrating the therapeutic equivalence of a generic product to the innovator product. Systematic and comprehensive innovator product characterization can be used to make generic product development easier. This involves characterization of API and quantification of the critical excipients. The latter contributes towards performance of the final dosage form. This article describes the capsule formulation of a poorly water-soluble drug, celecoxib, which contains sodium lauryl sulphate as a critical excipient. The importance of a decoding process aimed at developing a generic product that matches the innovator formulation in a discriminating dissolution method is demonstrated.

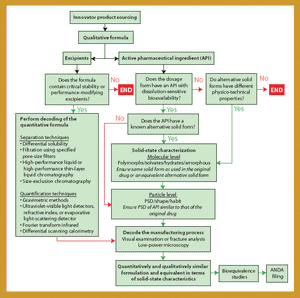
Being the first to gain the most is a fundamental principle in the generics business because several companies compete to create generics of successful products going off patent. For a generics company to maintain revenue growth in a market in which product prices continue to fall, it must secure a continuous flow of new products, with quality and speed to market being key drivers. Thus, generics companies must be highly skilled in product and process development (1), the generics business, and achieving bioequivalence-the most critical development area.
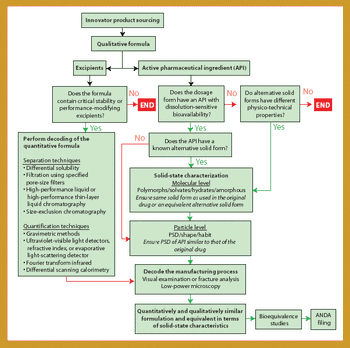
Being the first to gain the most is a fundamental principle in the generics business because several companies compete to create generics of successful products going off patent. For a generics company to maintain revenue growth in a market in which product prices continue to fall, it must secure a continuous flow of new products, with quality and speed to market being key drivers. Thus, generics companies must be highly skilled in product and process development (1), the generics business, and achieving bioequivalence-the most critical development area.

The authors examine the development and performance of coprocessed excipients, including testing them for flowability, compressibility, amd dilution potential.

The bioavailability and absorption of orally administered drugs can be improved by prolonging gastroretention through the use of floating, swelling, bioadhesive, or high-density systems.

The development of a successful powder for injection formulation requires careful study of preformulation parameters, packaging and process parameters, and the elimination of particulate matter.

Published: March 1st 2002 | Updated:

Published: July 2nd 2008 | Updated:

Published: March 2nd 2008 | Updated:
Published: June 1st 2006 | Updated:

Published: April 20th 2006 | Updated:
Published: August 2nd 2005 | Updated: