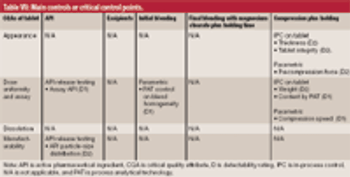
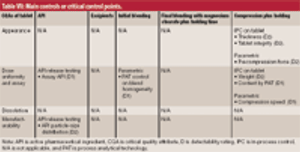
Criticality management combines pharmaceutical product, process, and material knowledge and risk management in one approach, which is reflected in a single document.
Filip Vanhoutte is an associate director of drug-product development at Johnson & Johnson Pharmaceutical Research and Development, Beerse, Belgium, tel. +32 0 14 60 39 58, fax +32 0 14 60 5333

Criticality management combines pharmaceutical product, process, and material knowledge and risk management in one approach, which is reflected in a single document.
Published: September 2nd 2008 | Updated: