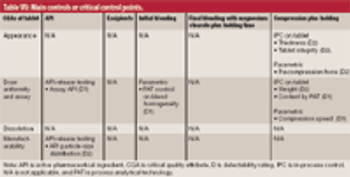
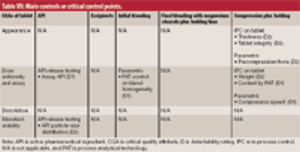
Criticality management combines pharmaceutical product, process, and material knowledge and risk management in one approach, which is reflected in a single document.
Luc Janssens is senior director of global regulatory affairs at Tibotec.

Criticality management combines pharmaceutical product, process, and material knowledge and risk management in one approach, which is reflected in a single document.
Published: September 2nd 2008 | Updated: