
Defining quality for excipients may not be as clear cut as it is for APIs, but it is essential to product quality and patient safety, and to choosing the best supplier.
Irwin Silverstein, PhD, is vice-president and chief operating officer at International Pharmaceutical Excipients Auditing, 1655 N. Fort Myer Drive, Suite 700, Arlington, VA 22209, tel. 703.351.5266.

Defining quality for excipients may not be as clear cut as it is for APIs, but it is essential to product quality and patient safety, and to choosing the best supplier.

The author discusses key areas of focus and presents best practices from the International Pharmaceutical Excipients Council (IPEC).

Choosing the right excipient manufacturer can help ensure the use of quality excipients.
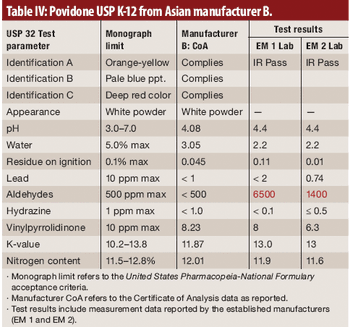
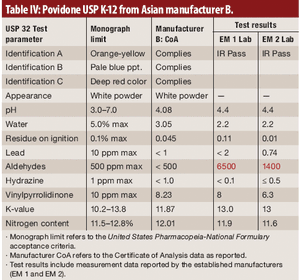
This article demonstrates that test results support the position of FDA on the importance of an appropriate supplier-qualification program.

The less complex nature of excipient manufacturers, as compared with API manufactures, carries many benefits.

A new set of proposed GMP standards for excipents may present more problems than benefits.
Published: October 2nd 2009 | Updated:

Published: May 2nd 2008 | Updated:

Published: June 1st 2002 | Updated:

Published: February 2nd 2016 | Updated:

Published: March 15th 2016 | Updated:

Published: July 6th 2016 | Updated: