
The authors explore and define common industry approaches and practices when applying GMPs in early development.

The authors explore and define common industry approaches and practices when applying GMPs in early development.

The authors explore and define common industry approaches and practices when applying GMPs in early development.
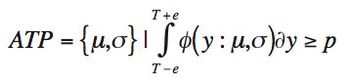
The quality-by-design principles used to control process variability are equally important to measurement systems because process variability includes contributions from measurement system variability. The authors use real-life examples from drug development projects to outline how an understanding of chromatographic measurement system variability might be achieved.

IQ Consortium representatives explore industry approaches and practices for applying GMPs in early development.

Published: August 2nd 2012 | Updated:
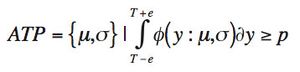
Published: September 2nd 2014 | Updated:
Published: November 2nd 2014 | Updated:

Published: December 2nd 2014 | Updated: