
This deal would help the companies merge to work on future projects and conduct research together.
Jill Murphy is Editor of Pharmaceutical Technology.

This deal would help the companies merge to work on future projects and conduct research together.

The first CleanCap analog was launched in 2017, and since then, the capping technology has been incorporated in one of the first commercially approved COVID-19 vaccines.

Forge will provide adeno-associated virus (AAV) process development, toxicology, cGMP manufacturing, and analytical services to Life Bio, which will occur at Forge’s gene therapy facility in Columbus, Ohio called the Hearth.

This approach is defined as being used to express transcription factor combinations to reprogram human induced pluripotent stem cells (iPSCs).

The objective of the partnership is to automate portions of ScaleReady’s CGT manufacturing workflow by using Cellular Origin’s robotic system for sterile liquid transfer.

The guidance is to provide information on compliance with applicable regulatory requirements and recommendations.

According to a press release, SGS has doubled its capacity for nitrosamine testing for North American customers.

The new portfolio from the acquisition will include contract research organization solutions, contract development and manufacturing organization solutions, and KSM/API solutions.

The new agreement is based off the Master Services Agreement that both companies entered in 2021 for the development, manufacture, and supply of rhenium (186Re) obisbemeda, according to a press release.

These models are useful in predicting whether an unknown substance of abuse can permeate the BBB to produce effects predicted by the Public Health Assessment via Structural Evaluation (PHASE) approach.

The recent revision is intended to provide guidance on QRM principles and tools that can be used for different aspects of pharmaceutical quality.

Presspart will still be responsible for the manufacturing of the Sunriser device.

mRNA therapeutics are being researched to support the fight against rare diseases, infectious viruses, and cancers.

This specific guidance refers to a DCT as a clinical trial where some or all the trial-related activities occur at locations other than traditional clinical trial sites.

The new research and development capacities set the stage for completion of product development for LEON’s manufacturing devices and will be used to showcase the devices and provide process development services to clients using LEON’s equipment.

The acquisition was completed through the merger of a wholly owned subsidiary of Sanofi with and into Provention Bio.

clonoSEQ Assay is authorized by FDA for MRD assessment in lymphoid malignancies and is highly accurate, sensitive, and standardized compared to other technologies used for disease burden assessment.

The results found that repeatability testing found all 15 processes to be with in specification for target final concentration.

Aspect Biosystems and Novo Nordisk A/S have announced a collaboration, development, and license agreement to develop bioprinted tissue therapeutics.

Jaeger notes that the program is focused on addressing specific scientific questions that will produce immediate impacts on how CDER and FDA make drug approval decisions.

The decision was issued in a joint statement by the FDA Commissioner and Chief Scientist, and effective on April 11.

The BalanCD CHO media portfolio is designed to ensure maximized growth, viability, and productivity of CHO cell lines.

Lon Johnson, VP of Sales and Marketing at Colbert Packaging Corporation, discusses the latest drug packaging updates and how companies can become more sustainable with Pharmaceutical Technology editor Jill Murphy.

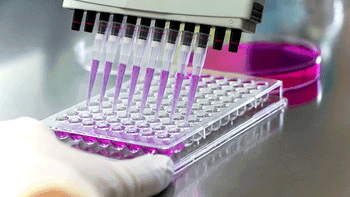

Contract testing services demonstrate a major increase in the speed and efficiency of testing using new testing methods and technologies.

The recent document will guide the use of DHT-derived data in regulatory decision-making for drugs and biological products.

Three new awards have been added to the roster for 2023 and include some specific highlights that have occurred over the past few years.

The combined solutions are currently available on the market.

The services are based on the company’s PurePrep EasyClean (PEC) orthogonal peptide purification technology.