
President Obama's economic recovery plain includes goals such as reducing the number of uninsured citizens and improving the quality of healthcare.

Jill Wechsler is Pharmaceutical Technology's Washington Editor, jillwechsler7@gmail.com.

President Obama's economic recovery plain includes goals such as reducing the number of uninsured citizens and improving the quality of healthcare.

Instead of protecting the public from unsafe drugs and contaminated foods, the Food and Drug Administration is a "hazard to public health," stated President Barack Obama in announcing his choices to head the agency and new efforts to improve food safety. Margaret Hamburg will be FDA's new commissioner, and Joshua Sharfstein principal deputy commissioner for drugs and medical products.

Broader disclosure of drug prices and conflicts of interest are central healthcare reform issues.

The stimulus bill expands the healthcare safety net while boosting investment in health IT and comparative research.

Efforts to cut healthcare outlays will focus on drug costs, despite a drop in prescription sales.
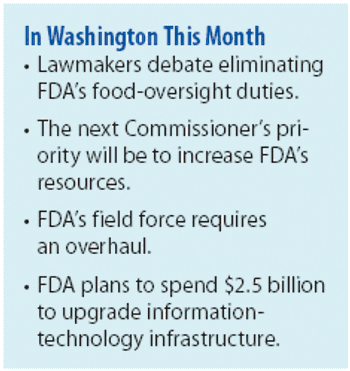
Will more resources and new leadership fix FDA, or is a major overhaul in order?

To expand coverage amidst the economic crisis, Obama will look for ways to cut healthcare costs.

The next president and the 111th Congress might change the extent of FDA's authority.

Policymakers weigh new rules to ensure the safety and quality of drugs made with tiny particles.

The US Food and Drug Administration seeks to understand nanotechnology better and exercise appropriate oversight over products that incorporate it.

Manufacturers seek real-world benefits from investment in QbD and risk-management strategies. This article contains bonus online-exclusive material.

FDA is taking several measures to ensure that imported drugs meet manufacturing standards.

FDA is modernizing adverse-event reporting as part of a revolution in drug-safety assessment.

New scientific discoveries promise to expand treatments for rare and neglected diseases.

Comparative-effectiveness analysis aims to promote appropriate pharmaceutical spending.

Regulators face demands to improve postmarket surveillance and meet review deadlines.

Heparin contamination casts a shadow on regulatory oversight of product quality.

Much to the surprise of most close observers of the Food and Drug Administration, Janet Woodcock agreed last month to resume control of the Center for Drug Evaluation and Research.

Quality-by-design submissions may reduce supplements and improve change management.

The rise in overseas manufacturing undermines FDA oversight of drug quality.

As FDA implements new drug-safety policies, manufacturers will focus on quality and pricing.

There are at least two major factors that will shape the manufacturing industry as a whole. Daniel Ruppar, Industry Manager (North America) for the Pharmaceuticals and Biotechnology Division of Frost & Sullivan predicts that, due to continued globalization, we can expect to see "growth centers for the industry move to emerging market sectors."

To keep up with applications, FDA promotes new testing and administrative methods.

New FDA act reshapes drug development and marketing to restore public trust in pharmaceutical regulation.

Expanded access to drugs for seniors has increased demand and focused attention on costs.

House and Senate leaders finally agreed on compromise legislation to renew prescription user fees, just a few days before the funding program was set to expire.

User-fee legislation will require more testing and data disclosure to prevent unsafe drug use.

FDA's long-awaited GMPs for supplements appear as food and drug safety concerns override lingering opposition.

The US Food and Drug Administration has pursued many new initiatives since 1977. The agency began accepting abbreviated applications for generic versions of drugs, collected user fees to support drug review, launched the Critical Path Initiative to encourage innovation, and worked to harmonize its regulatory standards with those of Europe and Japan. New challenges such as AIDS and bioterrorism have affected regulatory policy in recent years. The author reviews FDA's changes in policy and philosophy during the past 30 years.

Product characterization and production challenges are key issues in developing a pathway for biosimilar therapies.