
Fewer field offices and inspectors will increase reliance on manufacturers to ensure product and process quality.

Jill Wechsler is Pharmaceutical Technology's Washington Editor, jillwechsler7@gmail.com.

Fewer field offices and inspectors will increase reliance on manufacturers to ensure product and process quality.

FDA is buried in postapproval manufacturing submissions and seeks to reduce the scope of changes that require agency scrutiny.

FDA's new safety program, Critical Path Initiative, and user-fee proposal seek to reinvigorate pharmaceutical R&D.

Rockville, MD (Jan. 30)-The White House proposes a record $2 billion budget for FDA for the year that starts Sept. 30, 2007, an amount that consists of $1.6 billion in appropriated funds, plus some $450 million user fees for drugs, biologics, medical devices and other products.

"Quality by design" (QbD) and "quality risk management" at long last seem to be moving from the buzzword stage to becoming important influences on drug development and manufacturing. A series of quality standards issued by the International Conference on Harmonization (ICH) is encouraging the adoption of common quality-based drug manufacturing approaches designed to reach the "desired state" of drug manufacturing (i.e., more efficient, agile, flexible operations that can reliably produce high-quality drug products with less regulatory oversight). These developments reflect increased pressure to make pharmaceutical manufacturing more efficient and less wasteful and to encourage regulators in all regions to focus on the most critical issues affecting product quality and patient safety.

Democrats are back on top in Congress and are mapping a broad agenda for change. Prescription drug pricing, medical product safety, and access to needed treatments are high on the priority list. Manufacturers will be in the hot seat answering questions about patent practices, high-risk products, and why drugs cost less in other countries than in the United States. The real challenge, however, will be to gain approval of a bill to reauthorize the Prescription Drug User Fee Act (PDUFA) before the program expires on Sept. 30, 2007. Such legislation also would renew user fees for medical devices and continue the pediatric drug exclusivity program.

Washington, DC (Dec. 7)-One of the last acts of outgoing Senate majority leader Bill Frist (R-Tenn) was to push through confirmation of Andrew von Eschenbach as the official head of the Food and Drug Administration.

Brand-name manufacturers seek to protect their products.

FDA must monitor a growing number of facilities and complex drugs despite "resource challenges."

A recent analysis found that 9000 marketed drugs are not in FDA's National Drug Code Directory.

Washington, DC (Sept. 12)-The Office of New Drug Quality Assessment (ONDQA) in the Center for Drug Evaluation and Research (CDER) has approved one new drug application (NDA) under its CMC Pilot Program and has two more applications are under review. The pilot was established last year to provide an opportunity for FDA and industry to explore strategies for including Quality by Design (QbD) principles and process analytical technology approaches in regulatory submissions, explained ONDQA deputy director Chi-wan Chen at the PDA-FDA Joint Regulatory Conference here

Partnerships launched 63 research projects that may translate into nine or 10 new drugs by 2010.

The new drug user-fee program will define how FDA does business over its next 100 years.
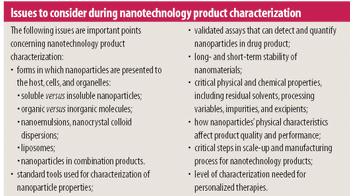
FDA is conducting laboratory research to understand better the ability of preclinical screening tests to identify potential risks and toxicities of nanotechnology-based drugs.
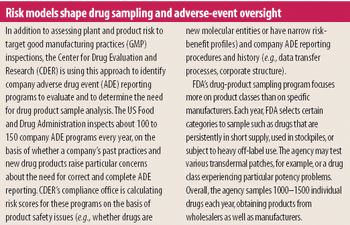
ORA leadership looks to staff redeployment and risk management to ensure product quality despite diminishing resources.

Manufacturing and formulation innovation spurs drug develop-ment, but raises new safety and quality issues.
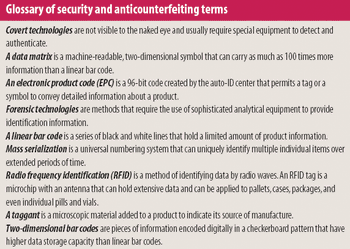
The need to curb drug counterfeiting is spurring development of track-and-trace and product authentication technologies.

Bush Administration Nominates von Eschenbach to head FDA

A new FDA policy emphasizes quality control over GMP compliance in producing clinical trial supplies.

FDA is proposing revisions to the drug user fee program while it weighs changes for drug safety initiatives and expanded vaccine production.

Manufacturers, FDA, and research organizations are collaborating on efforts to spur innovation and streamline drug production.

CDER is reorganizing staffs and developing new policies to encourage industry adoption of quality production systems.

New leadership must address political and scientific issues as the Medicare drug benefit alters manufacturer incentives.

von Eschenbach Drops NCI Hat…for Now

Ensuring access to quality medicines while ramping up production requires attention to supply chains, bioequivalence testing, and patent and regulatory issues.

Commissioner Crawford’s top priority is to restore public confidence in FDA oversight of drug safety and quality.

Although new drug development usually focuses on clinical and preclinical research, moving innovative products from clinical testing to market mainly involves overcoming manufacturing capabilities and production challenges. Ensuring access to consistently high-quality critical vaccines and therapies needed to counter bioterrorism attacks is a topic frequently debated. Product shortages are leading to policies that expand US drug and vaccine manufacturing and ensure that US regulatory and healthcare policies avoid erecting roadblocks to high-quality drug production.

Proposals to expand drug adverse event monitoring will publicize emerging safety concerns and strengthen post-marketing compliance efforts.

Officials at the US Food and Drug Administration are working with industry and academia to develop more efficient and reliable drug production processes that can ensure a consistent supply of high-quality therapies. A modern manufacturing system based on harmonized regulatory policies across global regions is critical for meeting public demand for safe and effective medicines, while also reducing production costs and eliminating waste.

FDA is finalizing guidances and enforcing compliance with manufacturing standards as part of its efforts to ensure drug safety.