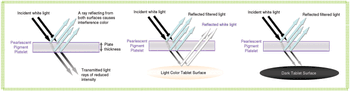
Sophisticated excipient development, especially for coatings, is staying on top of new challenges and meeting expanding industry needs.
See Maribel Rios's bio page.

Sophisticated excipient development, especially for coatings, is staying on top of new challenges and meeting expanding industry needs.

The US Food and Drug Administration has awarded the National Institute for Pharmaceutical Technology and Education (NIPTE), a not-for-profit organization comprising 11 universities, a $1.19 million contract to develop quality by design (QbD) guidance elements for design space and scale-up of unit operations.

This year, the employment survey will acknowledge the industry's best employers.

ISA 100.11a and WirelessHART both seek to become the global standard for industrial wireless automation.

Paul Sheskey of Dow Chemical provides an update on foam granulation technology.

Chemical imaging of solid dosage forms has become a powerful analytical tool for the development of solid dosage forms.

USP 467 Residual Solvents will take effect on July 1, 2008. But does the industry understand these specifications-and is it prepared?

The Parenteral Drug Association recently held its first global conference on emerging manufacturing technologies. Among the presentations were those discussing the latest in disposable technologies, aseptic filling and closing, and barrier systems. Clearly, technology vendors have stepped up efforts to streamline processes and better handle complex drug products.

Many companies are coming up with innovative materials and manufacturing methods to feed the growing demand for prefilled syringes.

As technology for impurity analysis improves, scientists are gaining better information and asking for more regulatory guidance.

Metrics Files Patent for Controlled-Release System

The findings of PTE/PT's survey of employment-related issues in the European and US pharmaceutical industries.

Emerging high-tech solutions prepare to cater to industrial automation with promises of increased efficiencies, faster communications, and custom applications.

Technologies ranging from new expression systems to PAT-inspired analyzers are driving biotech manufacturing forward to keep up with demand.

From data acquisition to enterprise resource planning, software systems operating at all levels of pharmaceutical manufacturing prepare for the seemingly inevitable implementation of process anlytical technology.

Despite global economic problems during the last 2 years and continued industry-wide lay-offs in the manufacturing sector, the pharmaceutical industry workforce continues at a slow and steady pace. Employees, however, seem ever more wary of their job security.

Despite continued industry-wide layoffs in manufacturing jobs during the past year, the pharmaceutical industry workforce continues a slow and steady pace.

FDA's aseptic processing draft guidance and the industry's state-of-the-art isolator technologies prepare manufacturers for the next generation of contamination control solutions.

Single-use filtration technology is becoming increasingly popular as manufacturers seek to cut costs and minimize processing times.

New research has led to significant advances in formulations and inhalation systems for pulmonary delivery.

After a year that has endured the devastating effects of global economic recessions, corporate bankruptcies, lay-offs and reorganizations, the need for a stable work environment has been steadily moving toward the top of every employee's list of priorities. Industry analysts have been carefully watching for signs of how these factors, and the responses to them from government and regulatory agencies, may ultimately affect the corporate job environment as a whole. The following results of the fifth annual international employment survey show that while the pharmaceutical industry remains strong, its employees are only cautiously optimistic.

After a year that has endured the devastating effects of global economic recessions, corporate bankruptcies, lay-offs and reorganizations, the need for a stable work environment has been steadily moving toward the top of every employee's list of priorities. Industry analysts have been carefully watching for signs of how these factors, and the responses to them from government and regulatory agencies, may ultimately affect the corporate job environment as a whole. The following results of the fifth annual international employment survey show that while the pharmaceutical industry remains strong, its employees are only cautiously optimistic.

More than 1600 industry employees report about the status of their current work environment, including salary, job environment, and outlook for next year.

Employees must understand the nature of the business they are in, the level of regulation that applies, and the consequences of noncompliance.

Schools of pharmacy are preparing their students to lead the industry forward by implementing infomatics, pharmacometrics, combinatorial chemistry, and engineering technologies in the classroom.

According to this year's survey, pharmaceutical employees working in Europe and in the united States are committing more hours to the job, earning higher salaries, and enjoying positive attitudes toward their current employment and outlooks for the future.

Published: December 2nd 2001 | Updated:

Published: May 2nd 2008 | Updated:
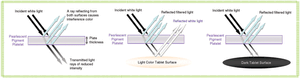
Published: November 2nd 2008 | Updated:

Published: October 30th 2008 | Updated:

Published: May 1st 2008 | Updated:

Published: February 2nd 2008 | Updated: