
Siemens enters agreement with Pfizer to commercialize diagnostic tests for therapeutic products across Pfizer's pipeline.
Melanie Sena is community editor of Pharmaceutical Technology.

Siemens enters agreement with Pfizer to commercialize diagnostic tests for therapeutic products across Pfizer's pipeline.

Moberg Pharma will acquire three OTC brands from Bayer HealthCare.

GSK forms the Oncology Clinical and Translational Consortium, a scientific research network comprised of six international cancer centers.

The University of Missouri's Department of Biochemistry and ABC Laboratories announce industry internship program.

Johnson&Johnson will open a network of partnering offices across the UK.

AstraZeneca will receive $76 million in damages from Apotex Corp., Apotex, and Torpharm.

Bristol-Myers Squibb has elected Thomas Lynch, Jr., MD to its board of directors.

ScinoPharm completes new API manufacturing plant in Changshu, China.

Rail-Mounted Fluid Bed Dryers Allow Cleaning Access
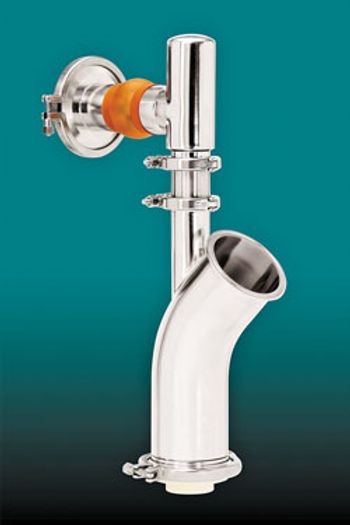
Low Feed Rate Feeder Designed for Pharmaceutical Processes

Tablet Tooling Coating Reduces Formulation Sticking

Bioreactor Functions as Perfusion Mimic

Whitehouse Laboratories announces the development of a Container Testing Center to open in 2014.

Sanofi discontinues development for its investigational JAK2 inhibitor, fedratinib.

GSK and Amicus Therapeutics revise Fabry agreement entered into in July 2012.

Elite Pharmaceuticals signs a manufacturing and license agreement with Epic Pharma for 12 generic drug products.

MedPharm opens a new topical and transdermal formulation-development services facility in Guildford, United Kingdom.

Penn Pharma partners with GEA Pharma to open a new facility for the development and manufacture of oncology drugs.

Kansas State University to build Bulk Solids Innovation Center in Salina, Kansas.

The US Pharmacopeial Convention posted for public comments on its revisions to the USP Medicare Model Guidelines, which are posted for a 30-day public comment period, beginning Oct. 1, 2013.

Fujifilm Diosynth Biotechnologies has renewed its manufacturing license from the UK's MHRA.
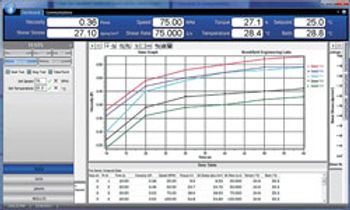
RheocalcT Software Tests Parameters and Data Collection
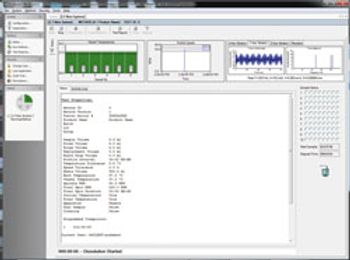
Software Improves Monitoring of Pharmaceutical Ingredients

3M introduces a rugged comfort respirator for harsh working environments.

Rockwell Automation releases a new guide to functional safety for process applications.

Ablynx and AbbVie sign license agreement for Anti-IL-6R Nanobody, ALX-0061 to treat inflammatory diseases.

Catalent Applied Drug Delivery Institute appoints Claus-Michael Lehr to advisory board.

Merck & Co. has received a Complete Response Letter from FDA for the resubmission of its new drug application for sugammadex sodium injection.

The CMDh agrees to suspend the marketing authorization of Numeta G13%E because of a risk of hypermagnesaemia.

The EMA Committee for CHMP recommends the approval of Xofigo in Europe.