
Merck KGaA (Darmstadt, Germany) plans to invest 190 million euros ($245 million) to build a biopharmaceutical plant in Darmstadt, Germany. Production is expected to begin in 2010.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

Merck KGaA (Darmstadt, Germany) plans to invest 190 million euros ($245 million) to build a biopharmaceutical plant in Darmstadt, Germany. Production is expected to begin in 2010.

Washington, DC (Aug. 14)?A new report issued by the Pharmaceutical Research and Manufacturers of America identifies 418 drugs and vaccines developed through biotechnology. All of the biotechnology medicines and vaccines are now in clinical trials or awaiting approval by the US Food and Drug Administration (Rockville, MD).

Teva Pharmaceutical Industries Ltd. announced that the company will cease production at its manufacturing facility in Cidra, Puerto Rico during the fourth quarter of 2006.

Novozymes (Bagsvaerd, Denmark) made an offer to acquire the the biotechnology company GropPep Ltd (Adelaide, Australia) for DKK 375 million ($65 million) to build its position in supplying ingredients to the biopharmaceutical industry. Novozymes is a major producer of enzymes, including biocatalysts used in pharmaceutical synthesis.

Akzo Nobel (Arnhem, Netherlands) is moving forward with plans to separate its pharmaceutical business into a separate company, Organon Biosciences

The US Food and Drug Administration has opened the FDA Electronic Submissions Gateway (ESG) to receive and process regulatory submissions to the Center For Biologics Evaluation and Research, the Center for Drug Evaluation and Research, and the Center for Devices and Radiological Health.

MSD Technology Singapore Pte Ltd., a wholly owned subsidiary of Merck & Co., Inc. (Whitehouse Station, NJ) dedicated an expansion of its production facilities in Singapore on July 28.

GlaxoSmithKline (London, England) has developed a vaccine against the H5N1 bird flu strain that, the company says, shows better immune response than other vaccines in development.

Combinatorial catalysis in asymmetric synthesis and asymmetric biaryl Suzuki coupling highlight recent advances in chiral chemistry. Plus the marriage of green and chiral chemistry.

Indian suppliers of active pharmaceutical ingredients and dosage formulations expand in India, the United States, and Europe.

Genzyme Corporation (Cambridge, MA) reports the US Food and Drug Administration (Rockville, MD) has approved the fill?finishing, packaging, and labeling of "Thymoglobulin" (antithymocyte globulin, rabbit) at its Waterford, Ireland facility. The approval allows Genzyme to begin manufacturing and distribution of Thymoglobulin from this facility.

Dietmar Hopp, cofounder of the German information technology giant SAP AG (Waldorff, Germany) is forming a new pharmaceutical company from the merger of two German biopharmaceutical companies: Axaron Bioscience AG (Heidelberg, Germany) and Lion Bioscience AG (Heidelberg, Germany).

Novartis (Basel, Switzerland) will build a cell culture-derived influenza vaccines manufacturing plant in Holly Springs, North Carolina. Construction is expected to begin in 2007.

In a move to strengthen its position in Western generic drug markets, Ranbaxy Laboratories Ltd. (Gurgaon, Haryana, India) acquired the Mundogen generic drug business of GlaxoSmithKline (GSK, London, England) in Spain, through Ranbaxy's Spanish subsidiary, Laboratorios Ranbaxy S.L.

Although the number of anti-infective vaccines (as distinct from therapeutic vaccines for cancers and other noninfectious diseases) entering clinical study each year since 2000 has been higher on average than it was in the 1990s, this product area may see little additional growth through the rest of this decade, according to a recentanalysis from the Tufts Center for the Study of Drug Development (Boston, MA).

The Bayer Group (Leverkusen, Germany) plans to sell the diagnostics division of Bayer HealthCare to Siemens AG (Munich, Germany) for EUR 4.2 billion ($5.36 billion).

Dutch biotechnology company Crucell NV (Leiden, Netherlands) and its technology partner DSM Biologics BV, a business unit of Royal DSM NV (Heerlen, Netherlands) will open a new research and development center that will specialize on further developing the "PER.C6" human cell line for the expression of recombinant pharmaceutical proteins.

MedImmune, Inc. (Gaitherburg, MD) reports that US Food and Drug Administration (Rockville, MD) has approved the company's supplemental biologics license application to use reverse genetics technology to construct new vaccine strains to produce seasonal influenza vaccines.

Select large custom manufacturers expand capacity, private equity firms buy companies in transition, and players from India and China build their positions.
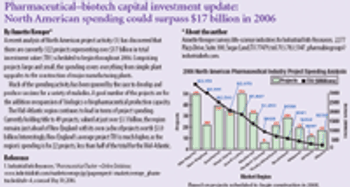
Pharmaceutical Technology provides perspective on pharmaceutical manufacturing activity, assessing the product portfolios, prescription volumes, revenues, and capital spending plans of more than 500 drug-makers. Novartis leads in diversity, with 889 products; Pfizer leads in number of scrips (352 million) and revenue ($44 billion). And generics companies are coming up on the inside.

The US Food and Drug Administration (Rockville, MD) approved the first generic versions of "Zocor" (simvastatin), the anticholesterol drug by Merck & Co., Inc. (Whitehouse Station, NJ).

Pfizer, Inc. (New York, NY) reports the United Kingdom's Court of Appeal has upheld the exclusivity of the main patent covering atorvastatin, the active ingredient in "Lipitor."

Johnson & Johnson Company (New Brunswick, NJ) agreed to buy the consumer healthcare business of Pfizer, Inc. (New York, NY) for $16.6 billion in cash.

Pfizer, Inc. (New York, NY) will phase out manufacturing operations in Groton, Connecticut, eliminating roughly 300 jobs.

Pall Corporation (East Hills, NY) reported strong sales growth in its biopharmaceuticals business in the fiscal third quarter (ending April 30, 2006) and announced plans for a company-wide cost-savings program.

Preformulation represents an early-stage opportunity to facilitate the eventual movement of a drug substance into a commercial product. Strategies to optimize the preformulation process were outlined by Harry Brittain, institute director for the Center for Pharmaceutical Physics (Milford, NJ). He spoke at the PharmTech Annual Event in Somerset, New Jersey this week.

The ongoing battle between Bayer AG (Leverkusen, Germany) and Merck KGaA (Darmstadt, Germany) in their respective quests to acquire Schering AG (Berlin, Germany) was resolved this week, with Merck KGaA agreeing to sell its 21.8% stake in Schering to Bayer.

NPIL Pharma, a subsidiary of Nicholas Piramal India Limited (Mumbai, India) has agreed to acquire the Morpeth, Northumberland, UK, manufacturing facility of Pfizer Inc. (New York, NY).

GlaxoSmithKline PLC (GSK, London, UK) will invest more than GBP102 million ($188 million) over the next four years in a vaccine manufacturing plant in Singapore.

Accurate characterization of active pharmaceutical ingredients (APIs) is a critical part of the drug development process. The approaches used to characterize APIs with respect to structure, identification of impurities, and the solid-state were discussed by Andrew C. Kolbert, manager, molecular structure and spectroscopy, Cardinal Health (Dublin, OH).