
If action is not taken within 60 days, the White House said it would “deploy every tool in our arsenal” to improve drug pricing practices for American patients.
Patrick Lavery is an Editor for the MJH Life Sciences brands Pharmaceutical Technology and BioPharm International, and their respective websites, pharmtech.com and biopharminternational.com. Previously at MJH, he filled the same role for LCGC International and Spectroscopy.

If action is not taken within 60 days, the White House said it would “deploy every tool in our arsenal” to improve drug pricing practices for American patients.

In this podcast episode, we discuss sustainable approaches to such equipment and materials as medical propellants, flow sensor technology, and walk-in chambers, plus the critical role of women in elevating and accelerating development in these areas.

7-OH and the leaf of the kratom plant are not analogous, but as the former is a derivative of the latter, it has opioid-like qualities that make its susceptibility to abuse concerning.

FDA’s latest whitepaper shows how scalable quality management investments in pharma reduce costs, improve reliability, and help prevent drug shortages.
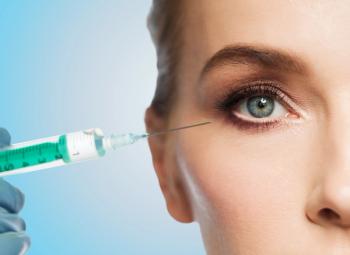
Re-Vana’s drug delivery technology is designed to release treatments over a 6- to 12-month period, with the goal of reducing frequency of injections for patients.

While a one-stop shop still has its advantages, sponsor companies and CDMOs are starting to see each other not as transactional relationships, but true partners with a common goal of getting drugs to patients faster.

Following an overhaul at ACIP, the HHS secretary took the advice of the committee’s new members, saying he was acting on guidance that dated back to 1999.

In this exclusive Drug Digest video, Deepak Bahl from Roquette and Jagruti Patel from Lonza look at strategies for accelerating early-stage development while reducing risk, approaches to speeding up timelines for complex formats without sacrificing quality, and maintaining a flexible CMC process that ensures quality.

The trade show is expected to draw 35,000 attendees to a show floor netting more than 1 million square feet.

Sotyktu (deucravacitinib) has been assigned a Prescription Drug User Fee Act (PDUFA) goal date of March 6, 2026.
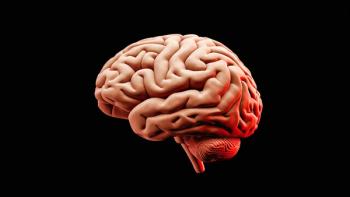
Delayed cerebral ischemia is a leading complication in the severe form of stroke known as subarachnoid hemorrhage and can result in long-term neurological damage, disability, or death.
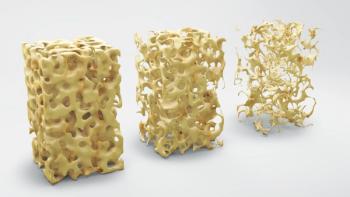
The company estimated that more than 1000 women in Scotland at very high risk of fracture would be eligible for treatment.

The expansion of the Kentucky site accounts for $80 million of the announced investment, with the remaining money earmarked for facilities in Michigan.

In contrast to earlier conferences in 2025, experts interviewed as part of the BIO conference in Boston did not come to a consensus about lasting impacts of recent changes in US government policy.

The partnership was formed in September 2024 in response to increasing demand for aseptic filling technologies and processes.
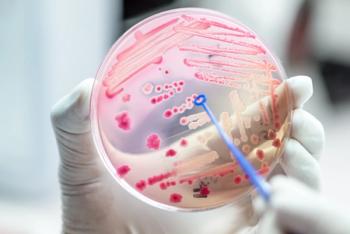
Prokaryotics will gain worldwide rights to develop, manufacture, and commercialize NAB741, a non-bioactive polymyxin designed to increase permeability of the outer membrane of Gram-negative bacteria.
The company’s mocravimod, a novel oral S1P receptor, is being evaluated for its efficacy as an addition to CAR-T cell therapies.

The department’s newly announced partnerships were part of a weeklong visit that included discussions held at BIO 2025.

NIBRT, Firefinch, and eg technology will join the center, adding to its collective of healthcare innovators.
The contest, which aims to accelerate the development of recombinant protein-based oncology therapies, will culminate at CPHI Frankfurt in October 2025.

Chrysalis lists among its partners early-stage biotechnology companies, contract research organizations, top-10 pharmaceutical firms, and various other life sciences stakeholders.

The collaboration will focus on identifying pre-clinical candidates for high-priority targets, including a small molecule oral therapy for immunological diseases.

The warning letters deal with a range of violations involving items from personal hygienic products to mismarketed analgesics.

The agreement has been designed to offer a turnkey service to companies seeking faster time to market.

NMPA’s Center for Drug Evaluation accepted Merck KGaA’s application for marketing authorization of pimicotinib as a Class 1 innovative drug for adult patients with TGCTs that require systemic treatment.

The partnership leverages the Hesperos organ-on-a-chip platform in the preclinical development of Psilera’s lead compound targeting the progressive neurological disorder for which treatment options are few.

Most of the company’s new additions, if not already online, will be scheduled for commissioning, validation, or qualification before the fourth quarter of 2025.

Smart technologies, such as digital and laser printing, incorporated into manufacturing equipment are helping manufacturers align with sustainability goals, according to Sheikh Akbar Ali, general manager and head of Development and Technology for ACG Packaging Materials.

The company describes the compact system as ergonomically designed, providing precision dosing and production floor flexibility.
Trends in certain forms of drug delivery, as well as the emergence of artificial intelligence, are playing roles in evolving the nature of partnerships, but there are new types of partnerships gaining steam as well.