
The most safe and effective therapies demand the highest data quality.
Peter Zipfell is product marketing specialist, CMD Software, Thermo Fisher Scientific.

The most safe and effective therapies demand the highest data quality.

The acquisition and analysis of MS data can be made more efficient with the integration of modern software tools.

The move to continuous manufacturing and Pharma 4.0 is resulting in a more data-driven approach to bio/pharma development. Enabling this approach are innovative data systems that can help users improve product quality while facilitating audit-trail review. First used with chromatography, they are now being applied to mass spectrometry.
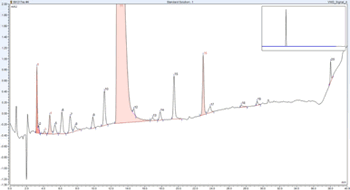
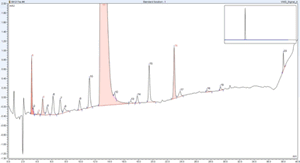
Integration of two separate chromatography data systems boosts workflow efficiency.

Published: March 2nd 2022 | Updated:
Published: December 5th 2018 | Updated: