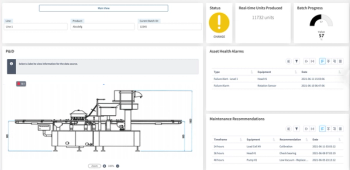
Aizon's Asset Health Application system is customized to manage each manufacturer's unique asset maintenance needs.

Aizon's Asset Health Application system is customized to manage each manufacturer's unique asset maintenance needs.

Linkam Scientific’s RHGen Relative Humidity (RH) controller allows for precise control of sample humidity without need for an external dry air supply.
New England Biolabs Luna Probe One-Step RT-qPCR Mix with UDG enables sensitive, linear, real-time detection of target RNA sequences.

FDA has revised the Emergency Use Authorization for sotrovimab.

AstraZeneca and Neuroimmune have closed an exclusive global collaboration and license agreement to develop and commercialize NI006.

BioMed X and Boehringer Ingelheim have announced the start of a new research project in brain sensor development.

Hovione has announced that Jean-Luc Herbeaux will take over for Guy Villax as CEO on April 1, 2022.

UCB has announced that it is extending its tender offer to acquire Zogenix.

EMA has recommended granting a marketing authorization in the EU for Kimmtrak (tebentafusp), a new medicine for a rare type of eye cancer.

EMA has recommended approval of Spikevax for children aged 6 to 11.

Johnson & Johnson has confirmed advancement of the nationwide opioid settlement agreement.

Pfizer and BioNTech receive positive opinion for COVID-19 vaccine booster in adolescents 12 through 17 years of age in the EU.

FDA approves Jardiance (empagliflozin) for wider range of patients with heart failure.

FDA licenses Certara’s Immunogenicity Simulator to research and evaluate immunogenicity in protein-based therapeutics.

AstraZeneca and Honeywell will partner to develop next-generation respiratory inhalers as part of AstraZeneca’s Ambition Zero Carbon program.

A new portfolio of chromatography and mass spectrometry consumables launched by Thermo Fisher Scientific is designed to meet higher performance requirements while remaining economical.

TFF Pharmaceuticals announces findings that inhaled niclosamide significantly inhibits viral replication of the Omicron variant of SARS-CoV-2.

Medicago and GSK have announced that Health Canada has approved COVIFENZ, an adjuvanted plant-based COVID-19 vaccine.

USP is developing mRNA quality guidelines to help companies and regulators bring innovative medicines to market faster.

Janssen will pay upwards of $1 billion for exclusive rights to use Remix’s RNA-based drug discovery platform on three specific targets.

Eli Lilly and Company are investing $700 million into a Boston-based facility for the newly announced Institute for Genetic Medicine.

Taconic Biosciences Cage+ colony management service aims to offer comprehensive coverage for contract breeding services.

MilliporeSigma and Waters will work together to build an extractables and leachables (E&L) reference library.

Scottish Enterprise has awarded £20 million (US$27 million) to Valneva Scotland to advance vaccine development.

Alvea has begun preclinical testing of a scalable, shelf-stable DNA vaccine against SARS-CoV-2 variants.

Moderna and Thermo Fisher Scientific have formed a collaboration to leverage dedicated commercial fill/finish manufacturing capacity in the US for mRNA vaccines and therapies.

Sanofi and GSK announce they are seeking regulatory authorization for COVID-19 vaccine.

Novavax announces the first doses of Nuvaxovid COVID-19 vaccine have begun shipping to European Union member states.

The Center for Breakthrough Medicines and BioAnalysis LLC have formed a strategic alliance to offer novel analytical testing services for CGT clients.

Semarion raises £2.14 million GBP (US$2.89 million) seed funding to support the commercial development of Semarion’s SemaCyte cell assaying platform.