
Company and People Notes: Catalent expands Bolton, UK, warehouse; Biotica appoints Edward E. Hodgkin as CEO and director; more.

Company and People Notes: Catalent expands Bolton, UK, warehouse; Biotica appoints Edward E. Hodgkin as CEO and director; more.

Bayer Schering Pharma officially completed the acquisition of Novartis?s biologics manufacturing facility in Emeryville, California.

Company and People Notes: Baxter and Halozyme Expand Relationship, Crucell Names COO, More.

Senators Chuck Grassley (R-IA) and Herb Kohl (D-WI) introduced the Physician Payments Sunshine Act to require pharmaceutical and biologics manufacturers to report the payments, gifts, honoraria, and trips they give to doctors.

The US Food and Drug Administration’s Interagency Working Group on Import Safety released a Strategic Framework based on a cost-effective, risk-based approach for ensuring the safety of products exported to the United States.

A team of researchers from the University of Idaho and Seoul National University have designed a potential drug delivery system by coating silica nanowires with the protein fibronectin.

Ranbaxy Laboratories has gained approvals from the US Food and Drug Administation to manufacture and market galantamine hydrobromide tablets and carvedilol tablets.

Company and People Notes: SAFC Expands in Wisconsin, Amgen Reduces Rhode Island Staff, More.

Boehringer Ingelheim decided to voluntarily withdraw its “Silomat” drug, which contains clobutinol hydrochloride, in all countries where it is available.
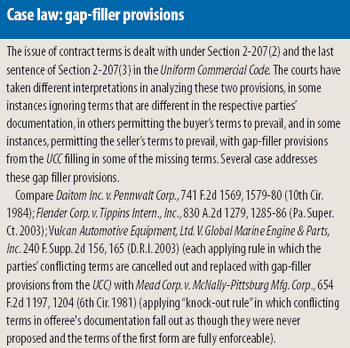
Purchase-order contracting is a commonly used approach to conducting commercial transactions, but it is a risky proposition when applied to pharmaceutical transactions, including the buying and selling of contract services and pharmaceutical ingredients. The authors examine the contract provisions covered in a commercial-supply agreement that are likely to be omitted under purchase-order contracting and the risk-reduction benefits that a commercial-supply agreement can offer in pharmaceutical procurement transactions.

US and China Crack Down on Regulation after SFDA Chief Executed; Xcellerex Receives US Grant for Biopharmaceutical Production; New FDA Guidance on Polymorphic Compounds in Generic Drugs; Comment Periods Open for ICH Q10 and Biologics Guidelines

Company and People Notes: GSK Hires Jacobs Engineering Group, Agilent Exec to Retire, More.

Catalent Pharma Solutions and Danish pharmaceutical company ALK-Abelló (Hørsholm, Denmark) will substantially increase Catalent’s “Zydis” oral-dissolving tablet production capacity dedicated to ALK-Abelló’s immunotherapy products.

The US Food and Drug Administration approved updated labeling for “Coumadin,” a common blood-thinning drug, that explains that patients’ genetic composition may influence their response to the drug.

August 23, 2007 Notes: AstraZeneca selects Axway's supply-chain tracking technology, Biovex names vice-president of quality assurance, more.

The US Food and Drug Administration announced it will hold a public meeting to discuss the safety and efficacy of over-the-counter (OTC) pharmaceutical products developed for the treatment of cough and cold symptoms in children.

Amgen announced it would cut 2200–2600 jobs as part of a plan to increase operational efficiencies.

Icagen signed a worldwide collaboration and licensing agreement with Pfizer for the discovery, development, and commercialization of compounds that modulate three specific sodium ion channels and could provide treatments for pain and related disorders.

Microbix Biosystems has established a team of experts to help manufacturers boost their production of influenza vaccine.

The US Food and Drug Administration announced the availability of a guidance document entitled “FDA Guidance for Industry: Exports Under the FDA Export Reform and Enhancement Act of 1996.”

Biomira Inc. and Merck KGaA update agreement, Tikvah names vice-president and CFO, more.

Senator Chuck Grassley (R-IA), a ranking member of the Senate Committee on Finance, asked the US Food and Drug Administration to explain what steps it is taking to ensure the safety of medicine manufactured outside the United States.

Commissioner Andrew von Eschenbach announced that the US Food and Drug Administration will not reform its Office of Regulatory Affairs until the Working Group on Import Safety finalizes its report evaluating import safety, according to the FDA News Drug Daily Bulletin.

Abraxis acquires manufacturing facility, Genentech acquires Tanox, more.

Johnson & Johnson consolidates operations, Xceleron leases new facility, more

Speakers introduced laser-induced breakdown spectroscopy, an analytical technique largely unfamiliar to the pharmaceutical industry, to attendees at this year’s Pharmaceutical Technology annual conference.

During a July 10 meeting in Lisbon, the European Medicines Agency and Heads of Medicines Agencies reviewed the achievements of the European Risk Management Strategy during 2005–2007 and the program objectives for the next two years.

Washington, DC (June 25)-More than five million US adults import prescription drugs from other countries, two million of them without an official prescription, according to a survey conducted by the Pharmaceutical Research and Manufacturers of America (PhRMA). Concerned about the number of counterfeit drugs entering the US, PhRMA launched the survey to determine who was importing prescription drugs and why.

A roundup of manufacturing and service expansions.

The latest pacts from the pharmaceutical supply chain.