
Nektar Therapeutics eliminated approximately 150 positions as part of a restructuring program designed to help the company complete its transition from a drug-delivery service provider to a drug-development organization.

Nektar Therapeutics eliminated approximately 150 positions as part of a restructuring program designed to help the company complete its transition from a drug-delivery service provider to a drug-development organization.

Amira and GSK Form Agreement, Xenome Appoints Ian Nisbet CEO, More...

Amgen will partner with R&D firm Takeda Pharmaceutical Company Ltd. toward the development and commercialization of 13 of Amgen?s molecules, with one included as an option.

The US Food and Drug Administration posted on its website a Jan. 24 Warning Letter to Novartis Vaccines and Diagnostics.

Brief pharmaceutical news items for February 2008.

A news roundup for February 2008.

Members of Congress have strongly urged the US Food and Drug Administration to reconsider its proposed rule to amend regulations permitting companies to promptly update their drug and device labels with new safety information.

Codexis Opens Budapest Lab, Allos Names Bruce K. Bennett VP of Manufacturing, More...

Connecticut Attorney General Richard Blumenthal is investigating Schering-Plough (Kenilworth, NJ) and Merck & Co. (Whitehouse Station, NJ), according to a Reuters report.

Actavis Acquires Site From Pfizer, Cardiokine Names President and CEO, More...

Roche and Ventana Medical Systems signed a definitive merger agreement, thus ending negotiations that began in June 2007.

Based on its investigation into the safety of over-the-counter cough and cold drug products for children, the US Food and Drug Administration is recommending against such products for all children under two years of age.

The US Food and Drug Administration published a draft annex to its ICH Q8 Pharmaceutical Development guidance that clarifies that document?s key concepts.

Novo Nordisk Drops Inhaled Insulin Product, Neurocrine Appoints CEO and President, More...

Noven Pharmaceuticals received a warning letter from the US Food and Drug Administration stemming from an on-site inspection of the company?s manufacturing facility in Miami, Florida, concluded July 2007.

The US Food and Drug Administration?s website will no longer allow the public to electronically submit comments to its dockets.

Pfizer and Hikal signed a contract manufacturing agreement in which Hikal will supply active pharmaceutical ingredients.

Crucell and Sanofi Pasteur Sign Agreement, WuXi Pharma Names COO, More...

The US Food and Drug Administration released a draft plan for modifying its information technology infrastructure. The plan follows the renewal of the Prescription Drug User Fee Act (PDUFA IV).

President Bush signed HR 2764, making appropriations to the US Food and Drug Administration during FY 2008, which ends September 30, 2008.

Merck Sells Facility to Cherokee Pharmaceuticals, Inflazyme Announces Senior Management Resignations, More...

Brief pharmaceutical news items for January 2008.
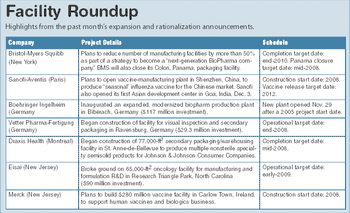
News, world briefs, facility roundup, events and other items for January 2008.

Millipore and Novozymes Form Alliance, Applied Biosystems Names President and CEO, More

Company and People Notes: West to reduce workforce, Eli Lilly's CEO and chairman to retire, more...

The US Food and Drug Administration posted on its website a Warning Letter it sent to Wyeth Pharmaceuticals regarding a journal advertisement for the company?s ?Effexor XR? antidepressant.

Company and People Notes: Eisai Acquires MGI Pharma, Ceregene Names CEO, More

Company and People Notes: Boehringer Ingelheim Expands Facility, Regulus Therapeutics Names President and CEO, More

Pfizer?s restructuring plans reportedly include major investments in outsourced manufacturing operations, production facility closures, and personnel reductions.

Congress Focuses on FDA Inspections of Foreign Drug Facilities; Medical Students Oppose Big Pharma's Influence on Campus; FDA Revises Postmarketing Reporting Requirements