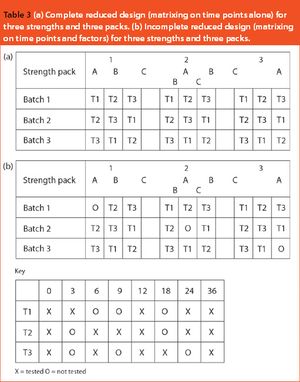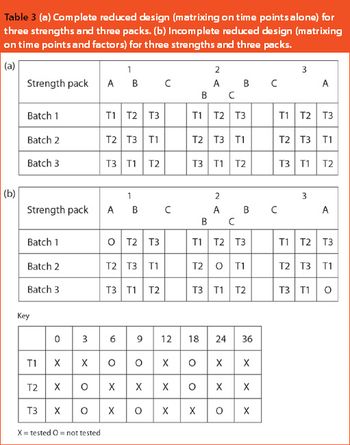
This article looks at the use of bracketing and matrixing to lower the number of stability samples required and, consequently, reduce the cost of sample production, testing and management. There is a common misconception that regulatory authorities will not accept such methods, but there is actually an International Conference on Harmonization guideline (ICH Q1D) on the subject. In fact, many of these designs have already been accepted and FDA members were among the first to describe matrixing.