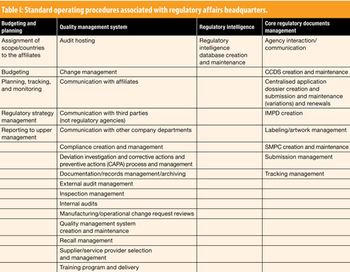
Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses standard operating procedures for the regulatory affairs department.
Siegfried Schmitt, PhD, is Vice President Technical at Parexel International, Siegfried.Schmitt@parexel.com.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses standard operating procedures for the regulatory affairs department.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how to report quality metrics to FDA.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how the regulatory requirements for CGMPs is the different phases of drug development and manufacture.
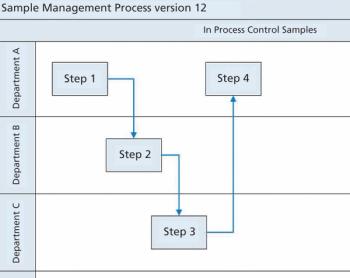
Siegfried Schmitt, principal consultant, PAREXEL, discusses how to streamline the document management process during market expansion.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to ensure archive records can be retrieved.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to assure compliance for automated systems.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to handle internal audit reports during inspections.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to keep up with changing regulations.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses good engineering practices.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how to handle staff challenges to regulation requirements

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses the requirements for good distribution practices.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses the benefits of automated processes.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how to ensure data integrity.

A drug sponsor?s responsibility does not end when a task is outsourced.

Siegfried Schmitt, principal consultant at PAREXEL, discusses the importance of quality-technical agreements.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how human error can be mitigated in pharmaceutical manufacturing.

Some recent high-profile cases of quality issues at Indian manufacturers have given reason to examine manufacturers more closely.

Siegfried Schmitt, principal consultant at PAREXEL, discusses the state of drug manufacturing in India.
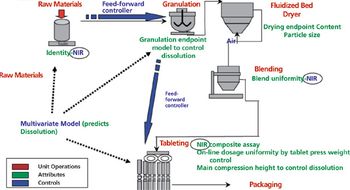
The authors present topics discussed and conclusions that resulted from the PDA QbD workshop.

There are no two completely identical freeze dryer units in operation anywhere.
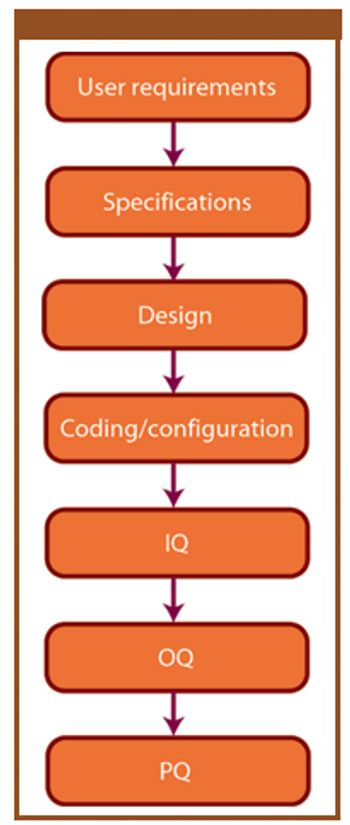
This article provides a historical review of computer validation in the pharmaceutical industry within the last three decades, evolving from the early years' initial concept and approach to today's current practices. Also included is how the regulations and industry have progressed in addressing the topic of computer validation.