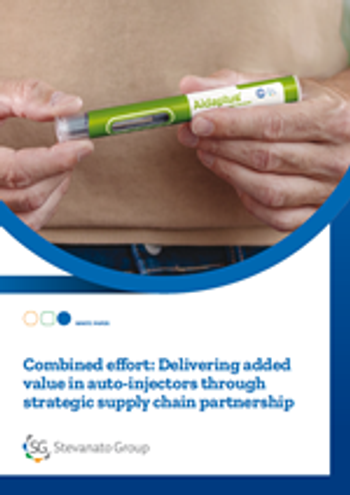
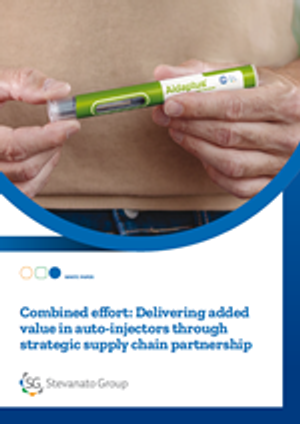
Stevanato Group’s integrated offering addressing mAbs challenges includes Aidaptus®, a 2-step single-use auto-injector accommodating 1mL and 2.25mL PFSs in the same base device, supporting a range of drug viscosities for reliable delivery.

Stevanato Group’s integrated offering addressing mAbs challenges includes Aidaptus®, a 2-step single-use auto-injector accommodating 1mL and 2.25mL PFSs in the same base device, supporting a range of drug viscosities for reliable delivery.

Don't miss this webinar delving into: - How to streamline the selection of the primary container and delivery device - How to overcome silicone challenges in PFSs - How to enhance the delivery of highly viscous drug formulation

The release of subvisible silicone particles and the risks associated with silicone oil droplets are major factors that can compromise the safety and efficacy of sensitive drugs. Download this white paper and discover how Stevanato Group's innovative syringe platform answers this challenge.

For new drug products, it is essential that pharmaceutical companies utilize the experience of container suppliers as early as possible. Read this white paper and discover how Stevanato Group can support you from start to finish.

Commercializing a drug-device combination product is a complex challenge to handle alone. Learn how Stevanato Group’s Technology Excellence Centers can provide guidance and execution through the various steps of a Validation Master Plan.

Innovative drug delivery solutions are evolving due to advances in biotechnology and the demand for biologics, focusing on user-friendly devices that improve patient compliance and support high-viscosity, large-volume treatments and various therapies.

There is not a syringe solution that fits all challenges. In this Podcast, Enrico Barichello, Product Manager for Syringe Platform in Stevanato Group, and Alan Xu, Product Manager for Analytical Services in Stevanato Group's Technology Excellence Centers, discuss the best strategies to select the right pre-filled syringe (PFS) based on the key needs of combination products and illustrate how introducing analytical services from an early stage can help customers reach the market faster by avoiding costly product revalidations or reformulations. Don’t miss out on the opportunity to be part of the conversation driving the PFS industry forward.

Enrico Barichello, Product Manager for the Syringe Platform at Stevanato Group, discusses the risks associated with the interaction between primary packaging and complex novel therapeutics and describes how to prevent or manage concerns utilizing newer, pre-sterilized syringe systems that have more unique characteristics.

Stevanato Group is a leading global provider of drug containment, drug delivery, and diagnostic solutions to the pharmaceutical, biotechnology, and life sciences industries.

Published: August 13th 2024 | Updated:
Published: October 16th 2023 | Updated:
Published: May 5th 2025 | Updated:

Published: May 5th 2025 | Updated:

Published: April 18th 2025 | Updated:

Published: May 5th 2025 | Updated: