
The review of batch records creates a story of the materials, manufacturing, and packaging involved in the production of bio/pharmaceuticals, according to Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

Susan J. Schniepp is a fellow at Regulatory Compliance Associates, Inc. and a member of Pharmaceutical Technology's Editorial Advisory Board.

The review of batch records creates a story of the materials, manufacturing, and packaging involved in the production of bio/pharmaceuticals, according to Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

Informing your clients of possible changes in equipment is imperative when upgrading a laboratory, says Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses the value of internal audits and how the information gained can be applied.

A robust quality agreement and good communication scheme can help avoid and alleviate regulatory concerns.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses how to ensure sterility when manufacturing small-scale parenteral batches.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses training personnel on a limited budget.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses the assessment of risk in the processing of intravenous injectable drugs.

Susan Schniepp, Distinguished Fellow at Regulatory Compliance Associates, discusses the regulatory requirements for improving manufacturing lines.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses training personnel on data integrity.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general counsel, both of Regulatory Compliance Associates, discuss requirements for successful product technology transfer.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general counsel, both of Regulatory Compliance Associates, discuss how to create a robust CAPA system and how to identify root cause.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general counsel, both of Regulatory Compliance Associates, discuss how to write standard operating procedures that hold up to audits.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general counsel, both of Regulatory Compliance Associates, discuss performing investigations of biological products.

FDA’s proposed guidance for quality metrics raises questions about quantifying the tangibles and intangibles of quality culture.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general council, both of Regulatory Compliance Associates, discuss the requirements for a successful corrective action and preventive action (CAPA) system.

PDA’s new technical report provides a template for bio/pharma companies to follow to establish a risk-based approach to prevent drug shortages.

A new technical report guides bio/pharma companies in establishing a risk-based approach for prevention and management of drug shortages.

Industry players and FDA evaluation possible quality metrics to improve manufacturing efficiencies and avoid drug shortages.

Industry players offer suggestions for quality metrics as FDA continues to try and solve the problem of drug shortages.

Establishing a well-defined training program is a crucial activity for any biopharmaceutical organization.

Companies can use metrics as a tool to help drive positive change and quality process improvements.

A thorough investigation of all possible causes of deviations should be performed.

Key talks from the recent PDA/FDA regulatory conference highlight room for improvement.
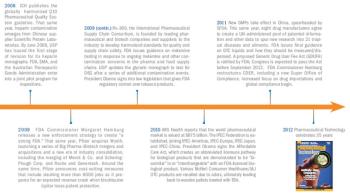
How FDA, USP, and ICH have redirected industry practice.

The contract provider needs to know as much as the NDA holder.

The author describes an equation that can be used to define the Quality relationship between a contract manufacturing organization and a client, including how to factor in both party's needs and regulatory commitments.

Contract organizations must have highly organized teams and plans to accommodate today's audits.

After a series of structural changes, the author wonders whether USP is undergoing an identity crisis. USP CEO Roger L. Williams responds.

Why SOPs are rarely followed, often cited, and in need of follow-through.

What to do when your CMO changes the manufacturing equipment line.