
Drug manufacturers have to be more than just "audit ready."

Susan J. Schniepp is a fellow at Regulatory Compliance Associates, Inc. and a member of Pharmaceutical Technology's Editorial Advisory Board.

Drug manufacturers have to be more than just "audit ready."
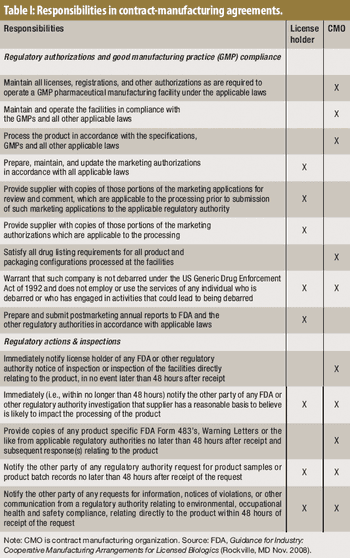
Before embarking on an outsourcing relationship, it's important to be aware of FDA's expectations.

Defining the Next Five Years.

The US Pharmacopeial Convention recently held its convention in Washington, DC.

New information improves an organization's guide to building manufacturing facilities.
We never thought implementing complex changes could become more cumbersome.

With a five-yar revision cycle around the corner, USP will hit or miss the collaboration mark.

Determined to prevent further supply-chain breaches, industry takes charge, offers proposals.

Standards data is helpful, but FDA needs to apply its information across the board. This article contains bonus online-exclusive material.

A new book inspires readers to seek ways to apply NMR spectroscopy to their own purposes.
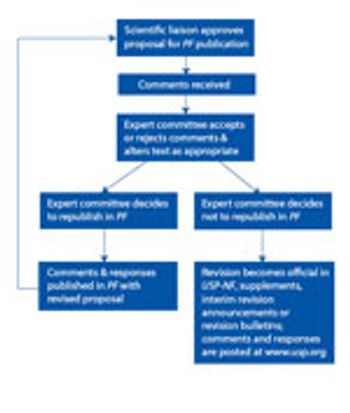
The USP public-comment process exists for a reason. Industry needs to take advantage. This article contains bonus online-exclusive material.
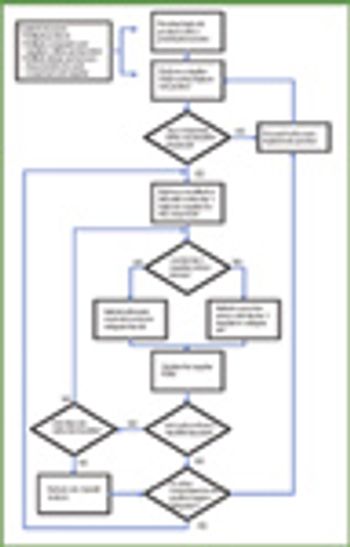
The growth and globalization of the pharmaceutical supply chain make risk assessment more important than ever for pharmaceutical manufacturers. The authors describe a program to identify, prioritize, mitigate, and communicate risks in manufacturer–supplier relationships.

Providing the right information upfront may ease new requirements to assess solvent levels.

Providing the right information upfront may ease new requirements to assess solvent levels.

The authors dispel common misinterpretations of the United States Pharmacopeia.