Diversifying the Global Heparin Supply Chain: Reintroduction of Bovine Heparin in the United States?
The global supply chain for bovine and porcine heparin and regulatory considerations are examined.
Tina S. Morris, PhD, is vice president of Biologics & Biotechnology at United States Pharmacopeia (USP)

The global supply chain for bovine and porcine heparin and regulatory considerations are examined.

A General Chapter on mAbs will be published in USP-NF as biologics increase their role in healthcare.

USP optimizes identification tests and impurities procedures.

USP promotes horizontal standards for biologics' quality attributes.
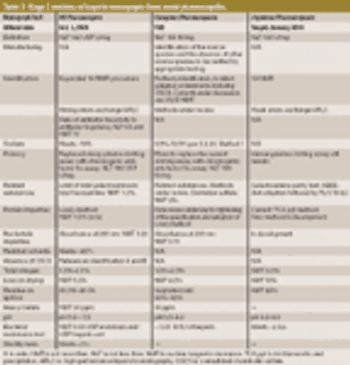
USP workshop participants support new methods to safeguard heparin products but desire international harmonization. This article contains bonus online-exclusive material.

USP's Stage 2 heparin monograph revisions address identification, potency, and impurities.

Published: September 2nd 2012 | Updated:

Published: August 2nd 2011 | Updated:
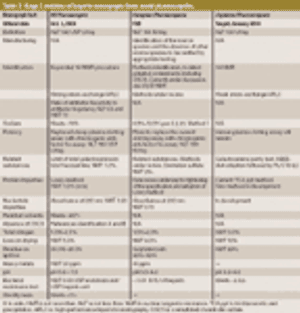
Published: October 2nd 2009 | Updated:

Published: March 2nd 2009 | Updated:

Published: March 2nd 2015 | Updated:
Published: November 2nd 2015 | Updated: