
Pharmaceutical Technology Europe
The authors present two concepts to improve robustness and facilitate continuous improvement in analytical methods.

Pharmaceutical Technology Europe
The authors present two concepts to improve robustness and facilitate continuous improvement in analytical methods.

Pharmaceutical Technology Europe
Although representing a huge market, the EU also presents problems that hamper profitability- particularly when it comes to differing price regulations across member states.

Pharmaceutical Technology Europe
With the pharmaceutical industry being in a current state of unrest in light of the recent spate of mergers, acquisitions, job cuts, divestments, patent expiries, and so on, we wanted to know how you felt about all these changes that will have directly or indirectly affected you.


Pharmaceutical Technology Europe
Statistics are often viewed as confusing and complicated, but multivariate data analysis (MVA) methods can be used to amass knowledge simply.

Pharmaceutical Technology Europe
Although once used only for large production processes, robotics are now working their way into every aspect of the pharma manufacturing processes.
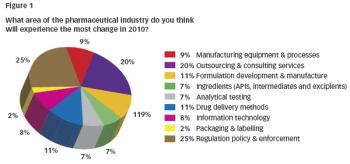
Pharmaceutical Technology Europe
Just before the end of 2009 we gave visitors to our website the opportunity to make some predictions about the 2010 pharma industry… and now it's time for the results.

Pharmaceutical Technology Europe
There is much scientific evidence of the early successes of whole cell therapies as disease cures in chronic conditions and disease-modifiers in acute conditions, but limited cases of successfully transferring these discoveries to commercial products or therapies.

Pharmaceutical Technology Europe
Pharma faces imminent expiries of key patents and increasing competition from generics. To protect what they already have, or what they will invent or acquire, companies must give patent protection the attention it deserves.

Pharmaceutical Technology Europe
Pharmaceutical Technology Europe discusses some of the latest challenges and innovations that are expected to define the future of pharma.

Pharmaceutical Technology Europe
PEGylation has been around for 30 years and it is surprising that it is still widely used given the significant advances that have been made in biopharmaceutical manufacture since then. So why is this the case?

Pharmaceutical Technology Europe
Last year, the UK's National Institute for Health and Clinical Excellence (NICE) decided that a new biopharmaceutical cancer drug costing £3000 per month, which could extend a patient's life by 6 months, was not value for money and would not be prescribed in the UK.

Pharmaceutical Technology Europe
The whole pharma/biopharma industry is under pressure to deliver cost-effective new drugs; however, the number of new drug applications is getting lower as the size of investment is increasing.