
Pharmaceutical Technology Europe
After a year of increased attention on the pharmaceutical supply chain in Asia, what will be the region?s short? and long?term role?

Pharmaceutical Technology Europe
After a year of increased attention on the pharmaceutical supply chain in Asia, what will be the region?s short? and long?term role?
Pharmaceutical Technology Europe
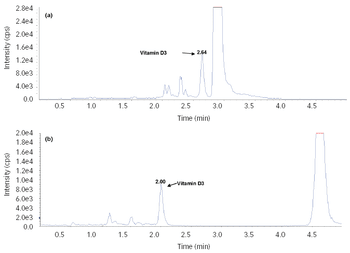
An LC–MS/MS method for the quantitative determination of vitamin D3 in human plasma has been developed and validated with positive atmospheric chemical ionization sources.

Pharmaceutical Technology Europe
Michiel Ultee talks about the challenges involved in transferring technology to a CMO and why communication is so important.

Pharmaceutical Technology Europe
The productivity of the pharma industry has been declining for several years. Could biotechnology reinvigorate drug development?

Pharmaceutical Technology Europe
This month's editor's comment.
Pharmaceutical Technology Europe
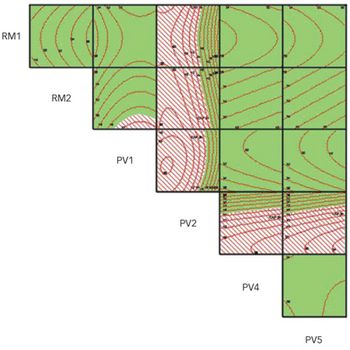
When applied as part of a structured approach, predictive modelling can provide deep process and product understanding, and can enable true, continuous process validation as envisioned by ICH guidelines.
Pharmaceutical Technology Europe
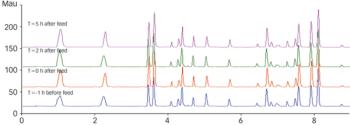
PAT guidance has been available from FDA for more than 4 years, but there have been no apparent breakthroughs in large-scale upstream production. Will companies consider using on?line chromatography to change this?
Pharmaceutical Technology Europe
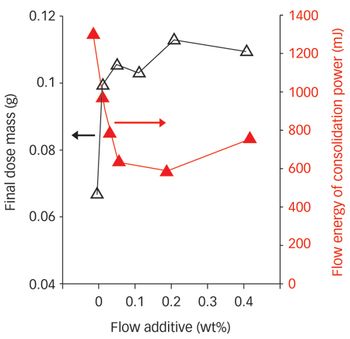
Enshrined in the concept of Quality by Design is the premise that optimized pharmaceutical manufacturing requires detailed understanding of products and processes. With this in mind, many benefits can be achieved by combining modern powder characterization techniques with real processing experience.
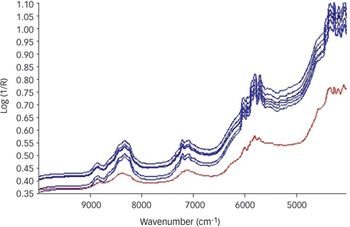
Pharmaceutical Technology Europe
This month's expert examines the most appropriate technique for checking raw material quality. What technique would you recommend for quality checking of raw materials?