
Pharmaceutical Technology Europe
Combination products have been researched and administered for many years, some successfully and others not.

Pharmaceutical Technology Europe
Combination products have been researched and administered for many years, some successfully and others not.

Pharmaceutical Technology Europe
In the current slow growth environment, service providers are looking to tactical expansions, as well as more ambitious strategic service expansions.

Pharmaceutical Technology Europe
Combining drugs with synergistic mechanisms of action yields some indisputable benefits; improved efficacy, reduced dosing, enhanced patient compliance, to name just a few.

Pharmaceutical Technology Europe
This case study describes the implementation of process analytical technology on the cultivation process step of a whole-cell vaccine against whooping cough disease.

Pharmaceutical Technology Europe
Increase your chances of success in the biggest market in the world.

Pharmaceutical Technology Europe
Parteck ODT is a newly introduced ready-to-use excipient for fast melt tablets.

Pharmaceutical Technology Europe
Belgium is one of the largest centres for pharmaceutical distribution and has the second highest number of pharma exports per capita worldwide.
Pharmaceutical Technology Europe
Human embryonic stem cells are of immense interest to researchers because of their ability to potentially develop into any kind of tissue.

Pharmaceutical Technology Europe
When validating automated systems from third-party providers, using the V model and failure modes effects and criticality analysis (FMECA) early in the process can help.


Pharmaceutical Technology Europe
Merger, union, alliance, partnership, collaboration: five words that ultimately suggest the strengthening of something.

Pharmaceutical Technology Europe
Why correctly calibrating a drug with its packaging is the key to success.

Pharmaceutical Technology Europe
The addition of superdisintegrants to oral solid dosage forms can improve disintegration and, in turn, drug dissolution.
Pharmaceutical Technology Europe
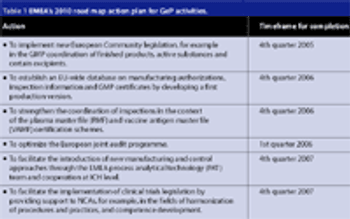
Since the formation of the European Union (EU) in 1993, each member state has brought along its own regulatory baggage, namely the standards and regulations that their companies are formally required to comply with. These standards and regulations still apply for any pharmaceutical products a native manufacturer decides to market within their homeland. When the same manufacturer markets its pharmaceutical products to consumers in other EU member states, the regulatory directives of the European Commission and the European Agency for the Evaluation of Medicinal Products (EMEA) apply as well.

Pharmaceutical Technology Europe
It's been a while since I last wrote my last editor's comment - a combination, during the last few months, of attending a variety of industry events (as the conference and exhibition season peaked before the summer) and being on holiday. I apologize for the poor excuses, but it's certainly better than saying the dog ate my homework.