
Pharmaceutical Technology Europe
Such an approach inevitably leads to increased front-end costs, but the advantages include better quality, reduced wastage and less product variability.

Pharmaceutical Technology Europe
Such an approach inevitably leads to increased front-end costs, but the advantages include better quality, reduced wastage and less product variability.

Pharmaceutical Technology Europe
Incorporating quality and economy into downstream purification processes can expedite first-in-human clinical trials, product licensure and technology transfer. Experience in the chromatographic purification of biopharmaceuticals enables the use of downstream processing heuristics to produce target molecules in cost-effective processes suitable for regulatory scrutiny.

Pharmaceutical Technology Europe
And so another year has passed; it seems that once you've reached the age of 30, your life accelerates at a very unnerving pace. Marriage, houses, kids ... they hit you all of a sudden and before you know it, you're reminiscing about your days at university - which of course, are now just a distant memory.
Pharmaceutical Technology Europe
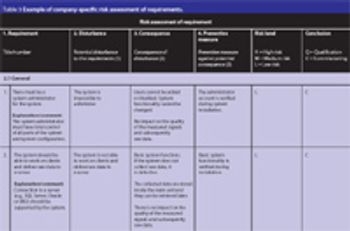
It is becoming evident that quality risk management within regulated, life sciences environments is a valuable component of an effective quality management system (QMS). A QMS provides a proactive and systematic means to identify, analyse, evaluate and control potential process and product quality issues during development, manufacturing, distribution and marketing throughout the entire product life cycle.

Pharmaceutical Technology Europe
A well-devised QPP, which has been agreed on and signed by both parties, saves time and makes it easier to complete activities such as design, installations and tests.
Pharmaceutical Technology Europe
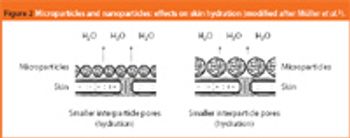
Lipid-based drug delivery systems - such as liposomes, micro-and nanoemulsions, self-emulsified drug delivery systems, and solid lipid micro-and nanoparticles - are becoming more popular because lipid materials are easily characterized, contain a high range of well-defined/tolerated surfactant molecules and can be developed for several administration routes.

Pharmaceutical Technology Europe
Never has greater pressure been applied to pharmaceutical manufacturers. Shelf space competition for branded drugs has reached aggressive proportions and now even prescription drugs vie for pricing and delivery. Against this is a backdrop of ever-increasing downward price pressures, and a spectrum of progressively more stringent legislative and quality requirements. Finally, regional markets now demand different tamper evidence technology, anticounterfeiting measures and safeguards against interference by biological terrorists. Much of which points to the need for innovation in packaging - not just in terms of pack styles and sizes, but also cost.

Pharmaceutical Technology Europe
In recent years, Big Pharma companies have shown increasing interest in setting up manufacturing in India and the Far East. The advantages of this outsourcing trend are obvious - decreased costs, coupled with ever increasing standards of operation. However, as the technological and quality of life aspirations of these new locations increase, the attractive cost advantages could dwindle and cause pharma companies to look elsewhere for manufacturing locations. Perhaps, they will consider Africa.