Pharmaceutical Technology Europe
How has pharmaceutical manufacturing validation influenced analytical instrument qualification during the last 20 years and what are the emerging trends for the future?

Pharmaceutical Technology Europe
How has pharmaceutical manufacturing validation influenced analytical instrument qualification during the last 20 years and what are the emerging trends for the future?

Pharmaceutical Technology Europe
The closing date for comments to be received by the US Pharmacopeia (USP) for proposed revisions to Chapter <231>, which deals with analysis of heavy metals, is 15 December 2008. The USP has been working towards for approximately 4 years and the task has not been easy.
Pharmaceutical Technology Europe
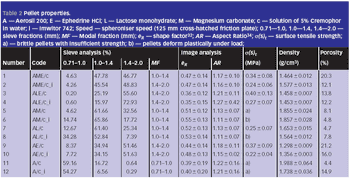
Microcrystalline cellulose is the main excipient used in the industrial manufacture of pellets by extrusion/spheronization, but pellets containing this spheronizing aid do not readily disintegrate and are expensive.

Pharmaceutical Technology Europe
New research suggests calcium carbonate tablets are stronger and less porous when manufactured using a wet, rather than a dry, granulation process.

Pharmaceutical Technology Europe
Hundreds of pharma–biotech deals have been announced during 2008 and many of these probably began at biopartnering conferences. Susan Aldridge examines some of this year's big buyouts and mergers.

Pharmaceutical Technology Europe
It is a universally acknowledged truth that a biotech start-up in possession of little fortune must be in want of venture capital (VC). Perhaps less well acknowledged, however, is that VC investors operate in a way consistent with being rational economic entities...
Pharmaceutical Technology Europe
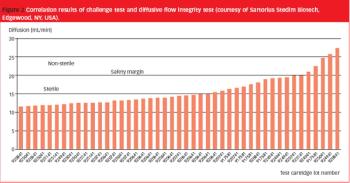
Although realized in 1983, ASTM's standrad test method for determining bacterial retention of membrane filters used for liquid filtration is still hugely beneficial to today's pharmaceutical industry.

Pharmaceutical Technology Europe
TranScrip Partners' Flic Gabbay explains why an approach that provides expertise on an 'as needed basis' is flourishing.

Pharmaceutical Technology Europe
...the new administration is not anti-science or even anti-industry. It just wants lower prices.

Pharmaceutical Technology Europe
You don't have to spend any time in the company of its chairman to see that there is no other Big Pharma company like Servier.