
As we approach the next instalment of Pharmapack Europe, a potentially perilous situation in relation to drug packaging has emerged for pharmaceutical companies should Brexit result in a ‘no deal’ scenario.

As we approach the next instalment of Pharmapack Europe, a potentially perilous situation in relation to drug packaging has emerged for pharmaceutical companies should Brexit result in a ‘no deal’ scenario.

The EU FMD deadline is closing in, so now is the time to look beyond the short term.

The new year has started with a bang in terms of mergers and acquisitions.

Active and intelligent packaging technologies benefit brand owners, caregivers, and patients.
Requirements for virus filtration must be considered in developing continuous downstream processes.

Charles Ross & Son Company has made improvements to the dual-post hydraulic lift and seal design of its 1500-gallon Multi-Shaft Mixer Model PVM-1500.

Fluid Air’s portable PolarDry Electrostatic Spray Dryer Model 0.1 is compact in size while retaining the same features of previous spray dryers.

The Epoch 2 Microplate Spectrophotometer from BioTek Instruments has added features that include monochromator-based individual wavelength selection or wavelength scanning from 200–999 nm to accommodate a range of assays, including nucleic acid and protein quantification, enzyme-linked immunosorbent assay, microbial growth, endotoxin, reactive oxygen species assays, and enzyme kinetics.

GEMÜ’s 1205 electrical position indicator is suitable for demanding applications in category 2, zone 1, and/or zone 21 ATEX areas, as well as for robust use at low temperatures down to -20 °C.

Orphan and cancer drugs continue to lead, but treatments for many common diseases were also approved in 2018.

Pricing pressures, investment volatility, and government disfunction greet Pharma in 2019.

This year promises to bring more focus on risk management and building a quality culture, says consultant Susan Schniepp.
This article demonstrates how qualitative physical attributes testing can be used to characterize soft gel capsule rupture/disintegration during rectal administration.

Understanding the differences in inspection processes is the key to successful global expansion, according to Siegfried Schmitt, PhD, vice-president Technical, PAREXEL Consulting.

FDA plans to support initiatives to ensure that all medicines are safe, effective, and of high quality.
Shortages of life-saving drugs are a regulatory and industry concern. Proper process development may help to ensure drug supply.
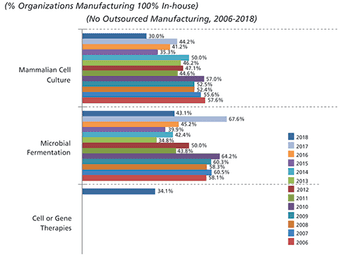
Outsourcing of manufacturing activities is expected to increase in 2019.

While pharma is proving its capabilities to develop novel therapies, the industry still needs to work on manufacturing innovation.

Keeping valuable employees happy-and on the job-may test bio/pharma business decisions.
Early adoption of the right approach to address solubility can deliver significant benefits.
New approaches seek to address formulation and delivery challenges for these complex molecules.

Click the title above to open the Pharmaceutical Technology January 2019 issue in an interactive PDF format.

As Brexit rapidly approaches, the lack of clarity around the regulatory balance between the regions could lead to a reduction in standards.

A brief preview of what to expect at pharma’s dedicated packaging and drug delivery event.

Intellectual challenge, work/life balance, compensation, and an unclear business outlook create uncertainty among European bio/pharma employees.

This coming year could see a combination of regulatory uncertainty and inactivity for the pharma industry, mainly as a result of Brexit.