
Can the pharmaceutical industry embrace the goals of Quality 4.0?

Can the pharmaceutical industry embrace the goals of Quality 4.0?

Contract partners must help innovators, especially smaller and virtual companies, consider manufacturability as early as possible in development. This requires focusing on technical and operational performance, as well as cost.
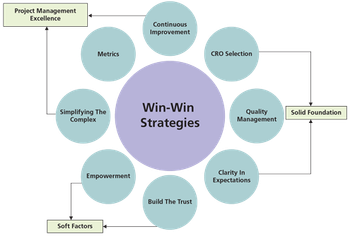
How to adopt win-win strategies and understand quality agreements for complying with cGMP when building strategic relationships with pharmaceutical contract research organizations.

CMOs and CDMOs expanded their services and facilities in 2019 and early 2020.

CDMOs must consider challenges associated with the complexity of contract pharmaceutical manufacturing when approaching digitalization projects.
As cell and gene therapies become more prominent, industry is seeking the expertise and capabilities of outsourcing partners to ensure success.

The need for manufacturing speed inspires contract manufacturers to explore advanced processing technologies.

CDMOs and CMOs will continue to invest in biopharmaceutical services and facilities as the bio/pharmaceutical industry looks to biosimilars and personalized medicine.

Click the title above to open the Pharmaceutical Technology Partnering for Bio/Pharma Success 2020 in an interactive PDF format.