
PAT: A New Dawn for Drug Product Quality

PAT: A New Dawn for Drug Product Quality

The stimulus bill expands the healthcare safety net while boosting investment in health IT and comparative research.

Without any GMP guidelines for excipients in Europe, change can't come soon enough for some industry groups.

GMP experts act fast to resolve some unusual and difficult problems.

The authors formulated and developed taste-masked RDFs of cetirizine hydrochloride for patients who experience difficulty in swallowing the tablet dosage form of the drug.

Brief pharmaceutical news items for February 2009.

The author defines sanitizers, disinfectants, and antibiotics, and examines the question of whether the rotation of disinfectants is scientifically warranted.
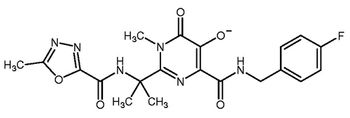
The pharmaceutical majors forward projects in biocatalysis, solvent replacement, and other approaches in green chemistry.

Dire circumstances are driving major drug companies to seek out CRO partners. This article contains bonus online-exclusive material.

Editors' Picks of Pharmaceutical Science & Technology Innovations
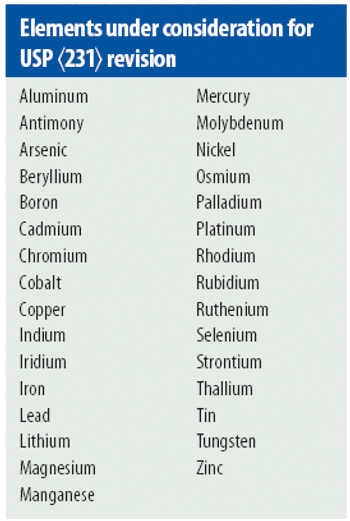
USP <231> Heavy Metals is transitioning toward the incorporation of modern quantitative technologies, but there is still much to be resolved.

To manage risk properly, industry must understand what it is and how to assess it

A recent reader poll focused on predictions for the industry's future.

The Romanian pharmacetuical market has grown significantly in recent years, but with stagnation on the horizon, a path forward is needed.

Efforts to cut healthcare outlays will focus on drug costs, despite a drop in prescription sales.

A new book inspires readers to seek ways to apply NMR spectroscopy to their own purposes.

FDA's role should not be overlooked as it has been in years past.