
Layout and supply details must be considered when implementing a fully disposable biopharmaceutical manufacturing process.

Layout and supply details must be considered when implementing a fully disposable biopharmaceutical manufacturing process.
A systematic framework and software are needed to implement material traceability in continuous pharmaceutical tablet manufacturing.

The following study describes a methodology to establish, control, and characterize an aerosolized bioburden within a test chamber and determine the settling rate of the bioburden.

New technologies, such as NIR and Raman, enable online measurements of blending and content uniformity in the production of solid dosage forms.
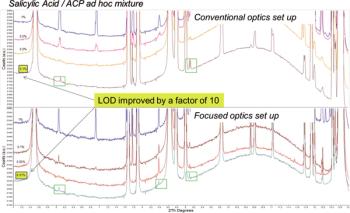
X-ray powder diffraction exploits the interaction between x-rays and matter to study the structural and microstructural properties of materials.

Experts share insights on the various methods used for purity and impurity analysis of therapeutic proteins.

The Flexicon PF7 peristaltic tabletop aseptic liquid-filling machine from Watson-Marlow Fluid Technology Group is suitable for GMP-regulated biotechnology and pharmaceutical operations.

Ross, Charles & Son's Tumble Blenders now offer protective light curtains that provide automatic safety shutoff whenever an operator crosses a defined security boundary.

Colorcon's Opadry SGR, Rapid Sugar Film Coating System, is an aqueous, high-gloss sugar film coating system for pharmaceutical products.
LogTag has added two new devices to its temperature data logger series.

Steve Hayward, product marketing manager at BIOVIA, Dassault Systèmes, discusses the importance of both technology and people in the modern laboratory.

This article explores lab data integrity violation trends, as well as a sampling of the latest technologies that can help avoid them.

Greater clarity and harmonization in ATMP regulations are needed to promote the development and commercialization of these therapies.

Hospitals form not-for-profit drug company to combat drug shortages and high prices.
Regulations and industry guidelines focus on ensuring excipient safety by specifying risk assessments and shared responsibility.

Proposed guidance documents assist drug manufacturers in qualifying single-use systems for commercial drug production.

While more companies are embracing taggants, researchers are developing new technologies that will be extremely difficult to reproduce.

Manufacturers tackle regulatory and competitive issues to develop complex therapies and biosimilars.

Regardless of the phase of development and the level of GMPs being applied, there should be adequate controls and knowledge to assure patient safety, according to Susan Schniepp, fellow at Regulatory Compliance Associates.

Click the title above to open the Pharmaceutical Technology February 2018 issue in an interactive PDF format.