
Measuring Spending Levels

Measuring Spending Levels

What to do when your CMO changes the manufacturing equipment line.

With a regulatory pathway for follow-on biologics, industry is wondering what FDA will do next.

The 2011 show presents ideas for package designs and equipment options for packaging lines.

New product reviews from March 2011.

Indian manufacturers are not a near-term threat to Western CMOs, but may be long term.
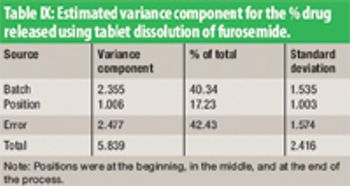
The authors evaluated the manufacturing data of 40-mg tablets of furosemide, a potent diuretic.

A revised book on process analytical technology could be valuable to novices and experts.

Brazil develops its first national plasma fractionation plant.
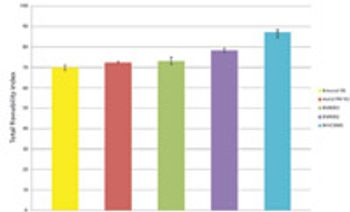
The authors investigated the tableting properties of PanExcea MHC300G, a high-performance excipient.

A single, global tooling standard would offer many benefits, but one has been slow to emerge.

The author describes key considerations for drug manufacturers when evaluating packaging partners.

Q&A with Robert Hardy, chief executive of Aesica

Social media tools have taken over many aspects of our lives, now including regulatory info.
A new audit guide aims to improve supply-chain security and supplier qualification practice.
The author provides an overview of QbD implmentation for biopharmaceuticals.

Just when things seem to be looking up, the unexpected problem occurs.
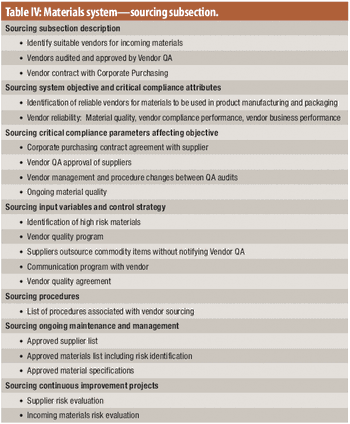
The authors review a compliance-by-design approach to quality systems.

Pharmaceutical Technology's annual survey on equipment and machinery reveals the spending levels and type of spending made in 2010 and planned for 2011.

FDA's efforts to improve access to treatments for rare diseases.

The power of emerging markets is reflected in the pharma's sales and production positions.

The author challenges current detection methodologies.

As drug shortages make headlines, FDA tests the Sentinel safety system and its efect on healthcare.

This report provides plans for the USP Council's work in the 2010-2015. This articles contains online bonus material.

INTERPHEX 2011 aims to address the industry's unique characteristics.