
Industry awaits the final revision of USP General Chapter and the impact it will have on the evaluation of sterile product package integrity.

Industry awaits the final revision of USP General Chapter and the impact it will have on the evaluation of sterile product package integrity.
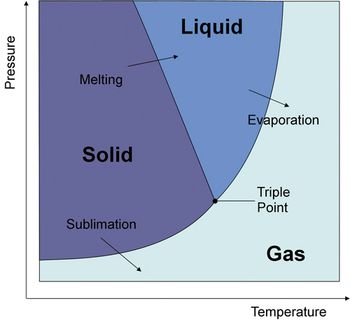
Efficient freeze-drying processes result in time and energy savings, reduced failure rates, and improved batch consistency.

Quality-by-design tools improve efficiency in scale-up of pharmaceutical processes.
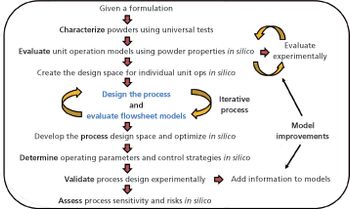
In-silico design facilitates process optimization and evaluation of process control strategies.

Meissner’s TepoFlex is a polyethylene biocontainer designed for secure handling and it is part of the One-Touch single-use systems portfolio.

Ross’ Discharge System with Electronic Pressure Control is designed for use with Ross Planetary Mixers and Multi-Shaft Mixers.
West Pharmaceutical’s sterile drug vial seal, the Flip-Off Plus, is manufactured using a production process that provides precise, consistent, and reproducible seals.

Quattroflow’s QF1200CV is a compact quaternary diaphragm pump designed for tabletop and cleanroom applications.

David Barrett, chief operating officer at cut-e, discusses The Behavioral Positioning System.

Drug developers understand the importance of early communication with regulators, but is EMA providing enough flexibility and support to companies?
Emerging controlled-release technologies could lead to more effective therapies in the near future.
The Human Microbiome Project has increased our understanding of the relationship between humans and microorganisms. The authors offer a new perspective on how this knowledge should be considered in setting standards for pharmaceutical quality control in microbiology.

In this article, industry experts discuss critical analyses for demonstrating biosimilarity.

Process design experimental data and risk assessments are used to predict expected process performance and establish process performance qualification acceptance criteria.

New legislation and changes in policy at FDA are leading to better control of the API supply chain.

FDA approves a biosimilar and loses a commissioner in March.

More reliable operations would accelerate product development and prevent drug shortages.

Hope abounds for local drug discovery companies despite challenges at home.
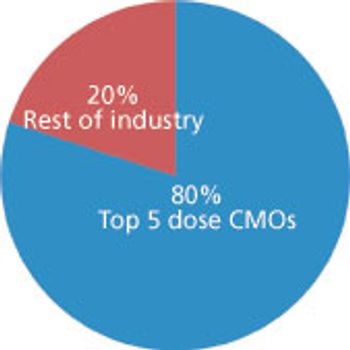
Big service providers get bigger faster thanks to Big Pharma.

Multivariate data analysis (MVDA) is being used to effectively handle complex datasets generated by process analytical technology (PAT) in biopharmaceutical process development and manufacturing.

Click the title above to open the Pharmaceutical Technology April 2015 issue in an interactive PDF format.