
Police and protests at BIO 2005.

Police and protests at BIO 2005.

The industry should develop a set of best practices for managing information technology systems and not wait for FDA to take the lead.
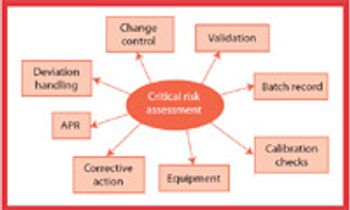
A stepwise, process risk-assessment approach can facilitate the identification and understanding of critical process parameters, quality attributes, and in-process controls. This approach can lead to more use of science- and risk-based regulatory practices to simplify the regulatory requirements for changes to synthetic processes and to support the underlying quality systems that ensure compliance.

Singapore is competing aggressively with India and China for a piece of the Asian sourcing business.

Able Laboratories, Inc. (Cranbury, NJ, www.ablelabs.com) announced in late May that it would halt all production and recall all available product because of problematic testing procedures discovered during an internal investigation. The company manufactures mostly generic prescription drugs, including drugs containing acetaminophen.

CA-Zoom 6.0 (Everest VIT)

Proposals to expand drug adverse event monitoring will publicize emerging safety concerns and strengthen post-marketing compliance efforts.

Can macromolecular processes learn from small-molecule experience? Burdened by exploding bioreactor productivity, architects of downstream bioseparation technology are looking into the drug industry's past for inspiration, while small-molecule companies adopt techniques pioneered by biotechnology. (The first of three articles on the current state of separations.)

At Convention 2005, USP's constituents ratified resolutions to continue and expand USP's international activities.