
Down the Track: Different Speeds with Multiple APIs

Down the Track: Different Speeds with Multiple APIs
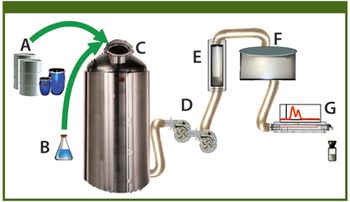
The author provides a review of PAT and tools such as near infrared analysis that may facilitate the use of PAT in the biopharmaceutical sector.

A joint biopharmaceutical manufacturing facility in India by Kenwell and Boehringer Ingelheim ushers in new era.

Formulators and manufacturers have many options for modifying release profiles in multiple-API products.
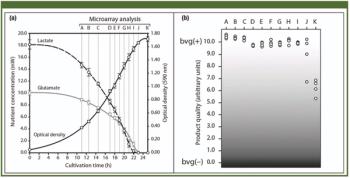
This case study describes the implementation of process analytical technology on the cultivation process step of a whole-cell vaccine against whooping cough disease.

Pan coating has been the preferred method of coating tablets for more than 20 years, but core coating is becoming more popular.

REMS to improve the safe use of opioids may lead to controls on other high-risk medicines.

The regulators are doing it. But industry's fear of sharing information may leave them behind.

Dramatic increases in yields from cell lines and the increasing popularity of single-use technologies will change the way we look at future bioprocessing facilities and process designs.
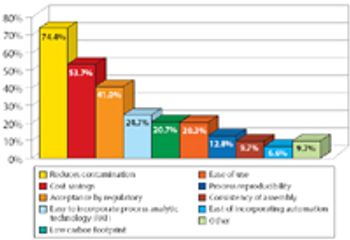
A Pharmaceutical Technology report looks at trends in biopharmaceutical manufacturing. This article contains bonus online-exclusive material.
Editors' Picks of Pharmaceutical Science & Technology Innovations
The authors modified gellan gum using microwave technology and showed it can be used as an excipient in tablet formulations.
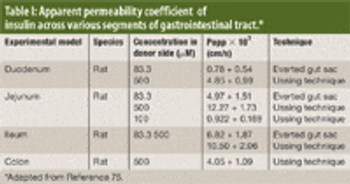
The authors review various oral drug delivery systems that have been explored to increase patient compliance for insulin.

While regulators begin to address nano-based drugs, industry should get its risk data ready.

Some GMP agents seem to find a way to squander time, money, and common sense.

Is it good policy to pay for bad behavior?
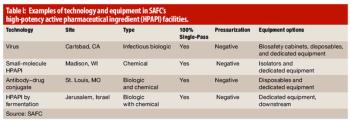
High-potency manufacturing of active pharmaceutical ingredients is a growing and specialized capability.

Traditional Chinese Medicine is widely used, but questions persist regarding its regulatory status.

Brief pharmaceutical news items for July 2009.

A look at the true cost-drivers of cell-culture production.

Developers of low-dose drugs in solid oral dosage forms will find theoretical considerations and practical advice in a new book.
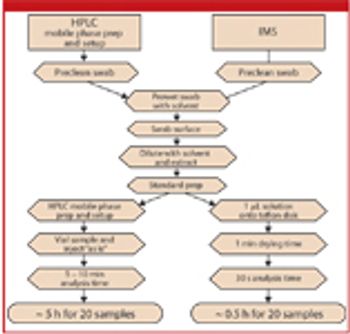
The authors discuss the theory of ion mobility spectrometry, its benefit over HPLC analysis in cleaning verification, and the experimental considerations for method validation and validation.
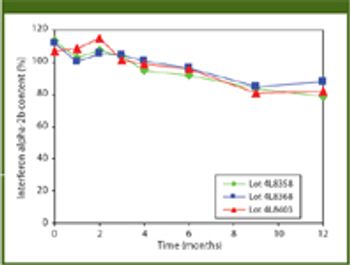
The authors describe a proprietary process for producing a stable, topical interferon alpha-2b formulation that can deliver large drug molecules into the skin or mucosa.
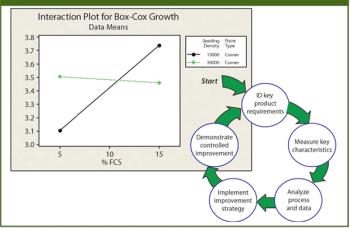
Large-scale manufacturing of human stem cells for therapeutic use is a leap in technology and science for the current biotechnology industry.
The authors discuss how strategic outsourcing to contract manufacturing organizations that have technical and regulatory expertise can add further value during vaccine development.
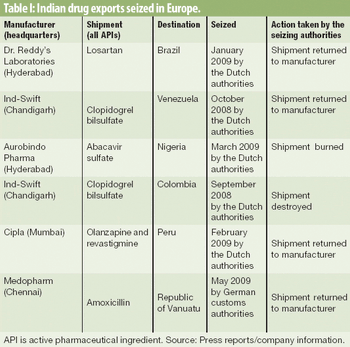
Patent infringement claims and a lack of clear global trade distribution routes may be unraveling the country's generic-drug export industry.