
Compounding, tracking legislation moves forward

Compounding, tracking legislation moves forward

The author discusses the purpose of analysis and testing and the implications for specifications and their underlying statistical distribution.
Osmotic systems offer versatility for delivering drugs with varied properties and dosage requirements.

Claudia Roth, President of Vetter Development Service USA, discusses trends in single-use technology for clinical manufacturing.

Brazil offers opportunities and challenges for global pharmaceutical companies.
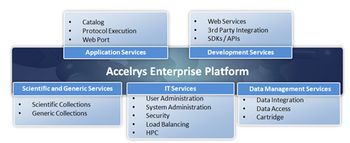
Editors' picks of pharmaceutical science & technology innovations; the latest in automation/IT/process control from Invensys Operations Management, Novatek International, Aspen Technology, and Accelrys.
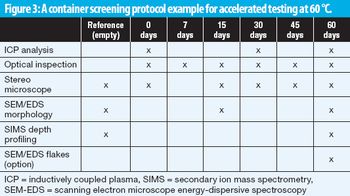
A screening method aligned with USP 1660 guidance predicts glass delamination in primary packaging for parenterals.

David Elder, vice-president, technical at PAREXEL, discusses the best way to productively participate in a regulatory meeting with FDA.

European governments are under pressure to take regulatory action, but solving the problem of medicine shortages is not as straightforward as it seems.

USP focuses on building worldwide partnerships in standards-setting activities.

Contract service providers expand capabilities in API and finished product manufacturing to meet demand for high-potency drugs.

The industry may not be ready for India and China as regulatory issues emerge.

Increased manufacturer outsourcing requires clear policies and written agreements with CMOs.

The author presents best practices for extractables and leachables.

The US and EU move forward with measures to fortify the pharmaceutical supply chain.

Pharma and biotech companies, with the rest of the health care industry, must face change.

Click the title above to open the Pharmaceutical Technology July 2013 issue in an interactive PDF format.