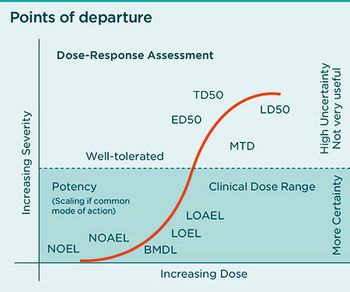
Determining how much containment is needed for API handling requires evaluation of multiple factors.

Determining how much containment is needed for API handling requires evaluation of multiple factors.

API can be mixed with silicone and other polymers to create drug-delivery combination products.

CMOs have been active over the past year in expanding their biologics production and capabilities.

This article provides a sampling of the latest investments, expansions, and acquisitions by small-molecule contract service providers.

Heightened uncertainty means CDMO executives need to play out planning scenarios.

Why shouldn’t biopharmaceutical manufacturers be able to leverage standardized construction practices to improve time to market?

A year’s worth of FDA warning letters suggest that API and finished drug manufacturers should strengthen their approach to continued process verification.
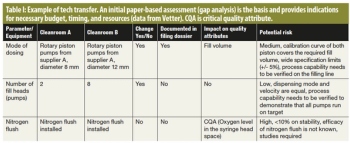
Real-life examples illustrate how to reduce the risks for each transferring partner and ensure that the development process meets regulatory requirements.

Click the title above to open the Pharmaceutical Technology 2018 Outsourcing Resources in an interactive PDF format.