
Soelkner discusses the latest industry developments and trends.

Soelkner discusses the latest industry developments and trends.
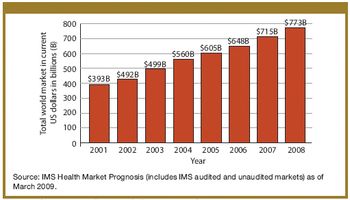
Biologics enhance their positions amidst slowing growth in the global and US markets.

South Africa was the first country on the African continent to become a PIC/S member. The country's Director of Inspectorate and Law Enforcement describes the 10-year process.

As the pharmaceutical industry looks to emerging markets, corruption becomes an important issue.
Editors' Picks of Pharmaceutical Science & Technology Innovations

Brief pharmaceutical news items for August 2009.

Biotech firms must first close the gaps between science and biology on the path toward QbD.
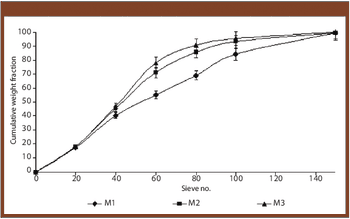
The authors prepared granules containing cinnarizine using polyethylene glycol 6000 as a melting binder and lactose monohydrate as hydrophilic filler. The effects of binder concentration and size were studied.

Big Pharma entered biotech too late. That same mistake can be avoided in personalized medicine.

An expanding number of products and services plug gaps in the cold chain. This article contains bonus online-exclusive material.

The author of an ambitious book about quality control falls short of reaching his goals.
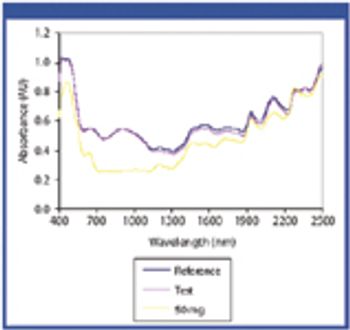
The authors applied near-infrared (NIR) spectrophotometry to assess whether eight drug products were authentic or counterfeit.

The author of a book about biopharmaceutical production includes irrelevant information.

Individuals and companies at the top seem to have no problem short-circuiting their success.

Determined to prevent further supply-chain breaches, industry takes charge, offers proposals.

Pressure to reduce healthcare spending has put drug rebates, price cuts, and tax hikes on the table.

An ounce of contamination usually leads to a mountain of investigation.

This article provides an overview of the drug Recall Root Cause Research (RRCR) project, an initiative of the US Food and Drug Administration's Center for Drug Evaluation and Research.

In the aftermath of recent restructuring, Big Pharma is sporting a reduced global manufacturing footprint while intensifying its focus to biologics and emerging markets. What will be the look of tomorrow's manufacturing networks?
View the corporate lineages of pharma's top companies.

China's State Food and Drug Administration has completed a new draft of GMP guidelines, but after a series of quality-control events, it will take time for the country to regain the global pharmaceutical industry's trust.