
Mid-size companies are forcing through their market positions by developing their niche competencies and their flexibilities to be recognized as serious market contenders.

Mid-size companies are forcing through their market positions by developing their niche competencies and their flexibilities to be recognized as serious market contenders.

The overall market size of the Southeast Asia region totals $7 billion, with a projected compound annual growth rate of about 13% through 2010.

For once, casting originators against generic players might end up strengthening the industry across the board.

As the pace of product development accelerates, the approach to dissolution-method development must advance beyond a manual method and an assay. A natural progression of the method-development process must include the transfer of the manual method onto automated instrumentation.

Before formal cleaning validation programs were instituted, visual inspection was the primary means of determining equipment cleanliness. The use of visual inspection is still typically a component of a cleaning validation program and for routine inspections of cleaning effectiveness, but the use of visual inspection as a sole criterion for equipment cleanliness has not been successfully implemented as a valid approach for cleaning validation.

I recently embarked on a quest: to investigate industry's use of the words, "generic" and "biosimilar" when describing a biologic molecule. An English major at heart, I was wrapped up in a news story that was partly about science, partly about words.
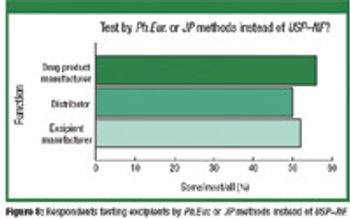
The Product Quality Research Institute (PQRI) conducted an open, publicly available, electronic survey of current excipient-control strategies among pharmaceutical excipient manufacturers, excipient distributors, and drug-product manufacturers (excipient users). Among the major findings are:
FDA will strengthen its collaborative relationships with federal agencies participating in the National Nanotechnology Initiative.

Potent-compound awareness training for operators is important to understand why the containment and controls are in place.

No matter how much we scamper about, the sheep just won't go where we want them to.

Apparently, the inspector would sneak off to visit his relatives on FDA time, instead of visiting us.
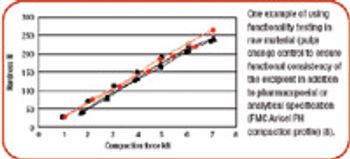
The debate over functionality is not about to be settled anytime soon.

A hybrid system using paper and electronic pedigrees will be needed.

Partnerships launched 63 research projects that may translate into nine or 10 new drugs by 2010.

Now is the time to get ahead of the learning curve and explore options for meeting pedigree guidelines.