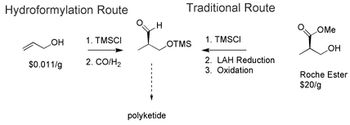
Researchers forward approaches for catalytic hydroformylation, asymmetric hydrogenation, and biocatalysis to achieve enantioselectivity.

Researchers forward approaches for catalytic hydroformylation, asymmetric hydrogenation, and biocatalysis to achieve enantioselectivity.

FDA must increase inspections of foreign API manufacturing facilities as more production moves offshore.

Contract manufacturers slim down to improve profitability.

Appendix: definitions and regulations, Federal Food, Drug, and Cosmetic Act; Appendix: definitions and regulations, Title 21 Code of Federal Regulations; Appendix: definitions and regulations, Compliance Policy Guides

A comprehensive book helps readers navigate the European drug-approval process.

News and Views

Going digital can produce high-quality, full-color labels at potentially lower cost.

User-fee legislation will require more testing and data disclosure to prevent unsafe drug use.
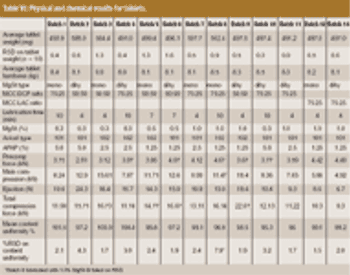
The influence of magnesium stearate (MgSt) on powder lubrication and finished solid-dose properties presents big challenges to drug manufacturers.

Jason Kamm, managing consultant with Tunnell Consulting discusses the challenges and opportunities for pharmaceutical manufacturers in ICH Q10, the draft guidance on pharmaceutical quality systems issued by the International Conference on Harmonization.


How the Indian pharmaceutical sector is reinventing itself

The draft guidance ICH Q10 for pharmaceutical quality systems is part of the ongoing move to a science- and risk-based approach in manufacturing.

In a nation of more than 1 billion people, the importance of vaccines goes beyond healthcare-it is a matter of national security. Armed with this belief and a philanthropic vision that most Indians could be protected against hepatitis, DT–Polio, and other afflictions, Dr. Varaprasad Reddy entered the nascent Indian biotechnology sector in 1992 and has since managed to threaten the monopoly of large laboratories. That year, the Hepatitis B vaccine cost $33 per shot, and yet some families were subsisting on less than $1 a day. Meanwhile, India was importing only 180,000 doses per year.

Useful Contacts

Poor processing and misguided projections lead to trashed product.

Robust module facilitates filter changes; Rupture disks provide high-volume flow; Auger feeder avoids cross contamination

In an age of "pre-existing conditions," can a new bill help patients get the treatment they need?

This article presents collaborative positions among excipient manufacturers, drug product manufacturers, and members of the US Pharmacopeia on key issues pertaining to the control of pharmaceutical excipients stemming from a recent Pharmaceutical Quality Research Institute workshop.

When it comes to research, the United States scientific community is still way ahead of the game, but we're not sharing our findings as often as we should. According to a new National Science Foundation (NSF) report, the number of science and engineering articles by US-based authors published in major peer-reviewed journals has plateaued.

US and China Crack Down on Regulation after SFDA Chief Executed; Xcellerex Receives US Grant for Biopharmaceutical Production; New FDA Guidance on Polymorphic Compounds in Generic Drugs; Comment Periods Open for ICH Q10 and Biologics Guidelines

Larger and strategic sampling and testing plans can improve process understanding and characterization.