
Creating closed processes and reducing room air classification in a biopharmaceutical facility can reduce operational costs.

Creating closed processes and reducing room air classification in a biopharmaceutical facility can reduce operational costs.

Operator attention to detail and adherence to procedures are crucial for proper cleaning.

Current guidance for absorption of elemental impurities does not address dermal exposure, resulting in a simplistic approach to limit setting.
Matt Shaffer, manager, formulation development, Bend Research, a division of Capsugel’s Dosage Form Solutions business, and Martin Koeberle, PhD, head of analytical development and stability testing, Hermes Pharma, spoke to Pharmaceutical Technology about the different approaches used in taste-masking and the challenges involved.
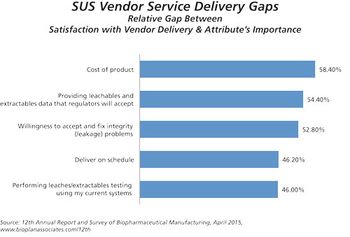
Suppliers indicate prices for single-use equipment are likely to increase.

Legislation to streamline drug development may get tangled up in user fee negotiations and drug pricing battles.

The industry is moving towards more flexible manufacturing with the use of modular facilities and single-use systems.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to assure compliance for automated systems.

EU and US regulators are striving to work together on improving GMP inspection efficiencies and avoiding duplication of efforts.

More complex drug candidates require more specialized and selective chemistry.

Industry Expert Q&A with Robin M. Silva, Partner, Morgan, Lewis, and Bockius LLP

Adents’ Pharma Suite serialization software features track-and-trace capabilities.

Kason’s Vibro-Bed fluid-bed agglomerator is equipped with imbalanced-weight gyratory motors and mounted on a spring suspension.
Meissner’s FlexGro single-use biocontainer assemblies feature the TepoFlex polyethylene (PE) multi-layer film and are delivered presterilized for immediate use.

Ross’ Three Roll Mill features hardened carbon-steel 52100 precision ground rolls, each cored for water cooling and heating.

Ensuring data integrity involves effort on an individual and global basis.

FDA’s proposed guidance for quality metrics raises questions about quantifying the tangibles and intangibles of quality culture.

QbD is improving the safety of solid-dosage drug products as well improving manufacturing processes, despite some industry reluctance.

Click the title above to open the Pharmaceutical Technology September 2015 issue in an interactive PDF format.