
In the pharmaceutical factory of the future, data collected by internet-connected manufacturing equipment improves operational efficiency.

In the pharmaceutical factory of the future, data collected by internet-connected manufacturing equipment improves operational efficiency.

Renovating a facility requires careful design and a plan to minimize production interruption.

Siegfried Schmitt, principal consultant, PAREXEL International, discusses how to find the root cause of the problem.

Industry experts discuss recent trends in modular manufacturing.

Manufacturers and regulatory authorities seek coordinated lifecycle management policies.

Companies may potentially accelerate the approval of generic drugs by avoiding deficiencies in ANDA submissions.
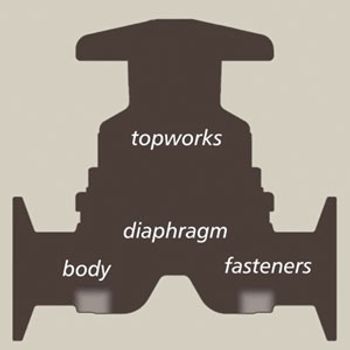
A process-specific preventative maintenance program improves productivity and reliability.

Tumble Blenders from Ross, Charles & Son now come standard with motors equipped with a electro-magnetic brake.

The BioProfile FLEX 2 from Nova Biomedical is a cell-culture chemistry analyzer that allows for automated cell-culture analysis for small-volume culture systems.

The OM-CP-CRYO-TEMP ultra-low temperature data logger from Omega Engineering records temperatures as low as -86 °C.

The FlowCam ALH automated liquid handling system from Fluid Imaging Technologies is a particle imaging and analysis system that automatically detects, images, and characterizes individual particles and microorganisms.
The media blitz surrounding drug shortages has stopped, but critical medications that have no substitutes remain in short supply. Can new approaches turn this situation around?
Nanoparticulate technologies are showing promising potential in delivering both small-molecule drugs and biomacromolecules across the blood brain barrier.

Visual inspection of parenteral vials is the first step in a root cause investigation.

Researchers at GlaxoSmithKline report a greener and lower-cost route to chiral fluorolactams that is suitable for scale up.

Swab recovery parameters are reviewed in detail to define best practices and highlight common mistakes to assure successful recovery studies using a risk-based approach.

The strategies of a innovation-driven CMO may be different than a capacity-driven CMO.

The authors of "Common Deficiencies in ANDAs for Dermatologic Drug Products" provide some examples of commonly cited deficiencies cited by FDA.

Click the title above to open the Pharmaceutical Technology September 2016 issue in an interactive PDF format.

The Parenteral Drug Association develops program to address barriers to implementation of postapproval changes.

Steep price increases for a popular drug have created patient and Congressional backlash.