
A multi-purpose biopharmaceutical manufacturing facility using a matrix of multi-functional cleanrooms can be adapted to efficiently meet the capacity challenges of both supplying clinical trials and launching products.

A multi-purpose biopharmaceutical manufacturing facility using a matrix of multi-functional cleanrooms can be adapted to efficiently meet the capacity challenges of both supplying clinical trials and launching products.

Senomyx uses an approach, known as taste-blocking, to address the challenges of bitter APIs. Kenneth J. Simone, vice-president of Pharmaceutical Business Development, Senomyx, speaks with Pharmaceutical Technology about this technology.
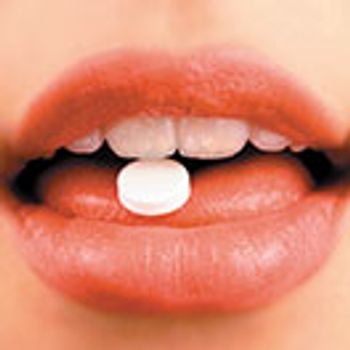
The level of tastemasking required will depend on the API properties and the dosage form design.

The European Commission’s proposed amendment on SPC waivers has sparked opposing views from drug originators and producers of generic drugs and biosimilars.

Watson-Marlow Fluid Technology Group added a new actuator suitable for applications where reduced weight is a concern.

groninger and SKAN have collaborated to develop Integra, a production line concept that integrates both an isolator and filling machine for vials with a large capacity range.

Ross, Charles & Son’s new mixing and discharging turnkey system is capable of extruding finished product into strands.

The SciLog SciPure FD System from Parker Bioscience is an automated single-use system for the filtration and dispensing of products into either bottles or bags.

Sharing know-how can help resolve common bio/pharma technical challenges.

Existing software tools cannot take into account the complexity of disease.

Increasingly complex trial protocols have added to IMP manufacturing challenges.

As counterfeiting, API manufacturing issues, and illegal diversion increase vulnerability, could dispensers and even patients play a greater role in securing the pharma supply chain?

This article focuses on applying new and traditional techniques to design a cleaning process, ensure the surfaces are clean, and develop rinse solution analysis to continuously monitor cleaning performance.

Single-use technologies, modular systems, and robots are on the rise.

Detailed process descriptions and robust documentation aid in compliance as well as training, says Siegfried Schmitt, principal consultant at PAREXEL.

Partnerships, mergers, and new services indicate that biologics are continuing to influence CMOs’ and CDMOs’ decisions to expand their biopharmaceutical services.

Click the title above to open the Pharmaceutical Technology September 2018 issue in an interactive PDF format.

The authors support the retiring of Ph. Eur. and USP heavy metal assays and propose a means of updating related specifications with minimal regulatory burden.

More consistent and reliable production processes are critical for advancing innovative treatments.