
CDMOs have established strategies for handling new chemical entities with unknown biological activity.

CDMOs have established strategies for handling new chemical entities with unknown biological activity.

Reeling from financial and tropical storms, Puerto Rico needs stable industry for recovery.

The pharmaceutical industry is under tremendous pressure to make drug development faster and cheaper. Applying the right formulation strategy using structured and rigorous science can help avoid costly failures and re-starts, but it’s important to start from an early stage.

Applying the right formulation strategies early in the drug development process can help avoid costly late-stage failures.

The European Commission is striving to tackle environmental pollution by pharmaceuticals as a means of curbing antimicrobial resistance.
Choosing between presterilized and bulk sterilized prefilled syringes.

Industry and FDA face new fee structures and new challenges in implementing fee initiatives.

Tools aid scale-up and comparison of single-use and stainless-steel bioreactors.
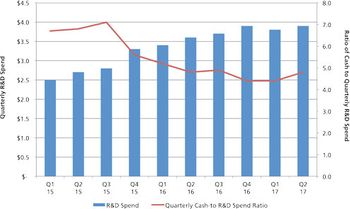
Mergers and acquisitions are positive for the CDMO industry, but there is a downside.

Focusing on whether the product meets its defined quality attributes should help one make reasonable, documentable, and defendable risk-based decisions, according to Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

The Thermo Scientific Pharma mini implant line is an integrated solution built around the Thermo Scientific Pharma mini HME twin-screw micro compounder.

Fluid Air’s PolarDry spray dryers use electrostatic technology, providing low-temperature spray drying, encapsulation, and selective agglomeration in the creation of particles.

Ross, Charles & Son’s VersaMix is a multi-shaft mixer designed for viscous applications requiring a high level of accuracy and batch-to-batch consistency.
An updated version of Shimadzu’s LabSolutions analytical data system incorporates additional functions to comply with data integrity regulations, and to support development and quality inspection procedures.

Capsules offer certain benefits over tablets for oral-solid dosage drugs, and several types of capsules are available.
Manufacturers introduce innovations in glass and plastic packaging for injectables.

EMA and FDA guidance encourages design of drug products to improve patient compliance.

Is there a difference between a specification and a standard?

This article discusses fully automatic inspection of glass and plastic containers and factors that affect particle detection rate.

This article seeks to promote dialogue among stakeholders to facilitate consensus regarding requirements for excipients.

The authors demonstrate that a proper measure of HPMC solution concentration is its electrical conductivity rather than its viscosity.

Just as FDA strengthens ties between reviewers and plant inspectors, proactive manufacturers are involving different disciplines in preparation for FDA audits.
Instead of rigidly applying statistical tools, experts suggest that pharma embrace statistical thinking, but focus on reducing variability and adding value for patients.

Lawrence Yu, deputy director of the Office of Pharmaceutical Quality (OPQ), Center for Drug Evaluation and Research (CDER), and his FDA colleague, Michael Kopcha highlight the importance of Six Sigma approaches to continuous improvement in the pharmaceutical industry.

Click the title above to open the Pharmaceutical Technology October 2017 issue in an interactive PDF format.