
Analytical and Bioanalytical Testing

Analytical and Bioanalytical Testing
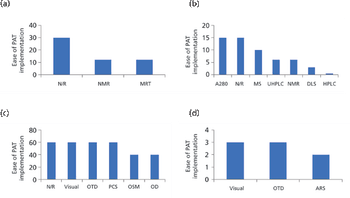
In this paper, the authors review the various analytical methods that can enable use of PAT.

New systems that combine Raman spectroscopy with automated imaging support the efficient gathering of such data, including information concerning size and shape distributions for individual components within a formulation.

Hydrogen deuterium exchange by mass spectrometry is a powerful analytical approach that can be used to map higher order structures of proteins.

This discussion aims to outline an approach to metal contamination prevention that should achieve a level of control acceptable to all stakeholders.
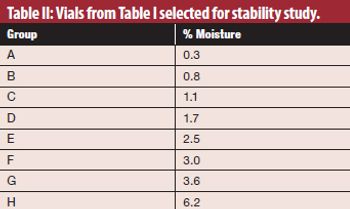
Using an alternate moisture-generation method may provide more accurate data for regulatory submissions.
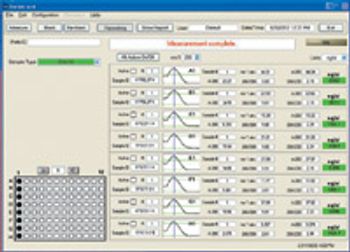
The authors describe a process for generating high affinity, fully human antibodies in culture.
This article focuses on the growing need for effective data management in the life sciences industry-especially among smaller pharmaceutical manufacturers.

Developing analytical methods and performing related testing is crucial for ensuring the quality of a pharmaceutical product.
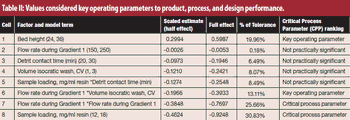
Critical process parameters (CPPs) and their associated process controls are crucial to drug development and process validation and to the evaluation of every manufacturing unit operation.
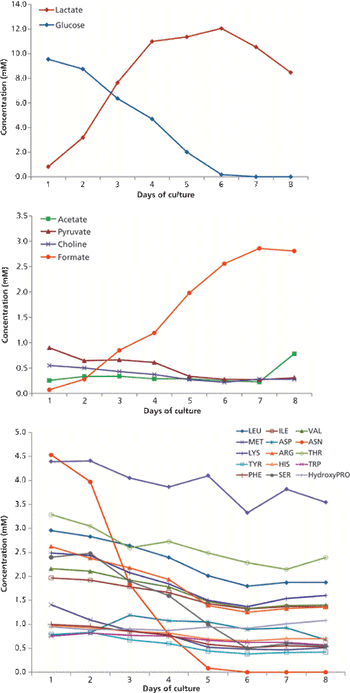
The author discusses current expectations in bioprocessing and lays a framework for using NMR to enhance a QbD approach.