
Kimberly-Clark Professional added the Kimtech A5 Sterile Integrated Hood and Mask XL for head shapes, sizes, and hairstyles that pose a challenge to standard aseptic gowning for cleanroom operators.

Kimberly-Clark Professional added the Kimtech A5 Sterile Integrated Hood and Mask XL for head shapes, sizes, and hairstyles that pose a challenge to standard aseptic gowning for cleanroom operators.

Boehringer Ingelheim plans to develop and test new strategies at its Solids Launch facility.

Transdermal patch design, materials, and manufacturing variables, as well as drug formulation and interactions between the API and the adhesive, can affect adhesion and drug delivery.

Virpax’s Patch-in-a-Can technology delivers pain medication using a metered-dose spray film.

To improve production in pharmaceutical manufacturing, the IT, OT, and Quality functional groups must work together to get the most value from existing plant data.

Drug and adhesive formulation are crucial to the development of microneedle patches for pharmaceutical transdermal delivery systems.

A required time frame should not be the driving force behind root cause investigations, says Susan Schniepp, executive vice-president of Post-Approval Pharma and Distinguished Fellow, Regulatory Compliance Associates.
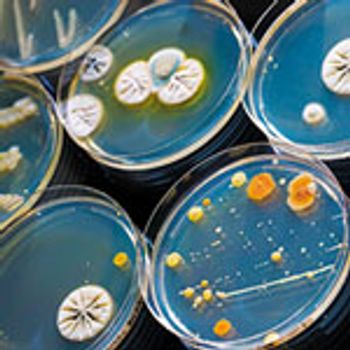
Microbial identity data can be critical for determining contamination sources.
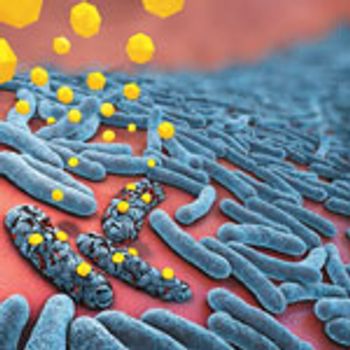
Antibody-based drugs offer new mechanisms of action and greater specificity.

Bio/pharma companies cannot spell success without solving all elements of quality programs.

Success depends on supplier communication and transparency, but it’s up to buyers to demand the right information and to look at the vendor’s overall business goals.

Simplified role-based training can lead to better quality metrics and compliance.

Once described as “throwing processes over the wall,” tech transfer is evolving into close collaboration and communication, as potential problems are considered sooner, and new technology is applied. Joseph Szczesiul, director of technical services for UPM Pharmaceuticals, shares best practices.

Drawing on practical experience, the authors examine key questions and answers about various aspects relating to the enhanced approach for analytical procedure lifecycle management.

Taking stock on the ‘big-ticket’ news items, both good and bad, from the past 12 months.

EMA’s relocation to Amsterdam and resulting staff losses could severely weaken the agency’s role as a leading medicines regulator.
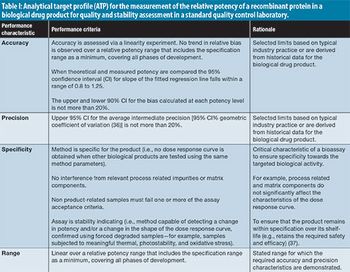
Three hypothetical analytical target profiles (ATPs) are provided, reflecting the current thinking of the the European Federation of Pharmaceutical Industries and Associations Analytical Lifecycle Management Team.
GMP non-compliance can spill over and impact patient access to life-saving medications.

CMOs and CDMOs made investments in new and expanded facilities and services in the last quarter of 2018.

A robotic tray-loading system from ESS Technologies gently handles glass prefilled syringes.

Charles Ross & Son Company recently developed two specialty customized 150-gallon double planetary mixers (Model DPM-150) with patented high viscosity blades.

Adents' DispaX offers improved supply-chain traceability for pharmaceutical warehouses, wholesalers, distributors, and dispensers such as pharmacies and hospitals.

Click the title above to open the Pharmaceutical Technology December 2018 issue in an interactive PDF format.
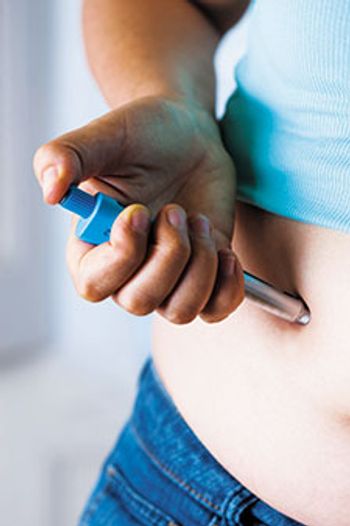
Using training devices may ease patient anxiety about using autoinjectors and prefilled syringes, potentially leading to improved patient adherence.

FDA Commissioner Scott Gottlieb has been promoting drug market competition in recent months that includes new guidance documents and targeted advisories to support R&D of complex drugs and combination products.

Despite ongoing efforts to address the problem, FDA now sees a rise in active shortages and in the duration of supply problems.