
Technological advances are helping shape the dosage forms of the future.

Technological advances are helping shape the dosage forms of the future.

Developers need to consider key challenges when approaching accelerated formulation strategies to ensure success.

Process performance metrics of eight different mechanical devices were assessed to evaluate compliance with regulatory and compendial criteria.
Bacteriophages could be crucial weapons in the fight against antibiotic-resistant bacteria.

Smart manufacturing transforms management of tablet and capsule equipment and processes.

A consensus-based approach to GDP lies at the heart of a new industry-wide program seeking to rationalize, standardize, and harmonize the adherence to pharma transportation norms and regulatory guidelines.
Scientists can work to overcome the challenges associated with protein characterization through empowering technologies.

Continuous improvements in technologies and services will help cold chain operators meet future industry demand.

Optimizing the use of partners for clinical trials depends on selecting the right contractor.

As Russia’s invasion of Ukraine continues, industry leaders speak out in condemnation of the actions taking place and pledge to provide aid to those in need.

The UK’s National AI Strategy underpins the government’s long-term commitment and ambitions to enhancing the country’s digital ecosystem, with the health and life sciences sectors seen as pivotal contributors to meeting these aims.

Califf will face challenges that include COVID-19, opioids, and user fees.

Borrowing is good, but invention is best.

Auditors must have access to the batch records of the activities they are reviewing, says Siegfried Schmitt, vice president, Technical at Parexel.

The ROSS X-Series Inline Ultra-High Shear Mixer produces quality dispersions, suspensions, and emulsions in a variety of industries.
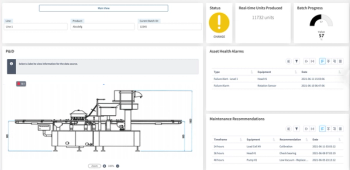
Aizon's Asset Health Application system is customized to manage each manufacturer's unique asset maintenance needs.

Linkam Scientific’s RHGen Relative Humidity (RH) controller allows for precise control of sample humidity without need for an external dry air supply.
New England Biolabs Luna Probe One-Step RT-qPCR Mix with UDG enables sensitive, linear, real-time detection of target RNA sequences.

Reducing US dependency on overseas pharma manufacturing may prove critical in navigating supply chain disruptions.

Although the bio/pharma supply chain has vulnerabilities that still need to be addressed, the COVID-19 pandemic has left lasting effects—some of them for the better.