
PTSM: Pharmaceutical Technology Sourcing and Management

PTSM: Pharmaceutical Technology Sourcing and Management
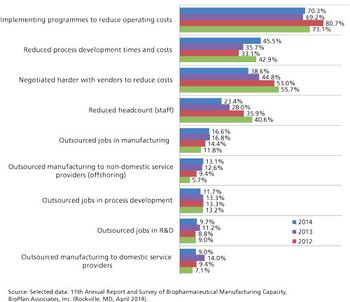
PTSM: Pharmaceutical Technology Sourcing and Management
CMOs are becoming sought-after partners as a result of their use of innovative technologies, single-use bioreactors, and other novel bioprocessing services.

PTSM: Pharmaceutical Technology Sourcing and Management
FDA’s Center for Drug Evaluation and Research implements its reorganization to bolster programs and policies to ensure drug with its “super” Office of Pharmaceutical Quality (OPQ).

PTSM: Pharmaceutical Technology Sourcing and Management
FDA delays enforcement of product tracing requirements to May 1, 2015, providing trading partners more time to comply.

PTSM: Pharmaceutical Technology Sourcing and Management
Analytical and procedural deficiencies result in FDA warning letter for Novacyl Wuxi Pharmaceutical.

PTSM: Pharmaceutical Technology Sourcing and Management
FDA set several milestones in approving more new, important drugs and biologics in 2014. Breakthrough drug designations went through through the roof, speeding more new therapies for cancer and critical conditions to patients.

PTSM: Pharmaceutical Technology Sourcing and Management
New organization offers support to contract research organizations and contract manufacturing organizations in the New England area.