PTSM: Pharmaceutical Technology Sourcing and Management
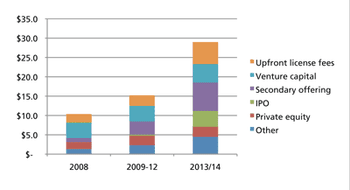
Memories of tough economic times may discourage CDMOs from expanding capacity.

PTSM: Pharmaceutical Technology Sourcing and Management
Memories of tough economic times may discourage CDMOs from expanding capacity.
PTSM: Pharmaceutical Technology Sourcing and Management
Early adopters look to speed up the use of continuous API manufacturing, but lack of expertise hinders adoption of continuous API synthesis.

PTSM: Pharmaceutical Technology Sourcing and Management
The investment doubles the company’s capacity to meet increasing demand for its biologics contract testing and biosimilar characterization services.

PTSM: Pharmaceutical Technology Sourcing and Management
Competitive pressures are driving more companies to repurpose APIs that had originally been developed for other indications. The industry has only "scratched the surface" of what might be possible, says consultant Hermann Mucke

PTSM: Pharmaceutical Technology Sourcing and Management
Teva Parenteral Medicines initiates voluntary nationwide recall of select lots of Adrucil due to particulate matter.

PTSM: Pharmaceutical Technology Sourcing and Management
Baxter has recalled of one lot of IV solution due to the potential for leaking containers, particulate matter, and missing port.

PTSM: Pharmaceutical Technology Sourcing and Management
“Rough notes” documentation and data management failures lead to warning letter for Mahendra Chemicals.

PTSM: Pharmaceutical Technology Sourcing and Management
Investment in Powdersize adds particle-size control solutions to Xcelience’s capabilities.

PTSM: Pharmaceutical Technology Sourcing and Management
FDA’s quality metrics draft guidance details the types of data the agency plans to request and the quality metrics it plans to calculate.

PTSM: Pharmaceutical Technology Sourcing and Management
EMA’s revised guideline on the implementation of accelerated assessment is open for public consultation.

PTSM: Pharmaceutical Technology Sourcing and Management
SafeBridge Consultants reports on the status of nine companies in the Potent Compound Safety Certification Program.

PTSM: Pharmaceutical Technology Sourcing and Management
Investment at Capsugel’s Edinburgh, Scotland facility will expand the company’s liquid- and semi-solid-fill capsule manufacturing capacity.

PTSM: Pharmaceutical Technology Sourcing and Management
Investment group Ardian will acquire the fine chemicals business activities of DPx Holdings B.V.

PTSM: Pharmaceutical Technology Sourcing and Management
World Courier receives pharmaceutical storage and transportation accreditation in the United Kingdom.

PTSM: Pharmaceutical Technology Sourcing and Management
CSafe and AES will support controlled-temperature shipping through the Basel, Switzerland airport from their new service center.

PTSM: Pharmaceutical Technology Sourcing and Management
The agency has released guidance on bioequivalence studies for asenapine, prasugrel, sitagliptin, and zonisamide.

PTSM: Pharmaceutical Technology Sourcing and Management
AMRI expands its API portfolio and European presence through acquisition of Gadea’s Crystal Pharma Group.

PTSM: Pharmaceutical Technology Sourcing and Management
Evans Analytical Group expands into the pharmaceutical/biopharmaceutical industry with acquisition of ABC Laboratories.

PTSM: Pharmaceutical Technology Sourcing and Management
Consisting of a needle hub and a needle shield, the new syringe closure system is partly produced by means of a two-component injection molding process of polypropylene and thermoplastic elastomer.

PTSM: Pharmaceutical Technology Sourcing and Management
CDMO for semi-solid and liquid manufacturing realigns operations at Texas facility.

PTSM: Pharmaceutical Technology Sourcing and Management
Charles Rivers strengthens its endotoxin testing and bacterial identification detection capabilities with the addition of Celsis’ products.