
PTSM: Pharmaceutical Technology Sourcing and Management
Mettler Toledo’s RX-10 Reactor Control automates jacketed lab reactors and controls thermostats, stirrers, and pumps.

PTSM: Pharmaceutical Technology Sourcing and Management
Mettler Toledo’s RX-10 Reactor Control automates jacketed lab reactors and controls thermostats, stirrers, and pumps.

PTSM: Pharmaceutical Technology Sourcing and Management
Flow chemistry systems from Dolomite Microfluidics can be configured to suit different applications.

PTSM: Pharmaceutical Technology Sourcing and Management
Shimadzu Scientific Instruments reports its EDX-7000/8000 spectrometers are compliant with electronic signature regulations.

PTSM: Pharmaceutical Technology Sourcing and Management
The SFT - HPR-Micro Reactor from Supercritical Fluid Technologies introduces a is a pressure reactor for small batch reaction chemistry.

PTSM: Pharmaceutical Technology Sourcing and Management
A high-pressure lab homogenizer from GEA for product development ensures efficiency and safety.

PTSM: Pharmaceutical Technology Sourcing and Management
Mayne Pharma plans to double oral-dose manufacturing space and expand contract services.

PTSM: Pharmaceutical Technology Sourcing and Management
After a long wait for the new elemental impurities guidelines, the bio/pharma industry must now look ahead to implementation and take action.

PTSM: Pharmaceutical Technology Sourcing and Management
Recipharm will be responsible for the supply of the remaining clinical-trial material and ongoing future commercial supply of RHB-105, the lead drug candidate developed by RedHill for the treatment of Helicobacter pylori bacterial infection.

PTSM: Pharmaceutical Technology Sourcing and Management
WuXi's Laboratory Testing Division will be the exclusive supplier of laboratory testing services for Hong Kong-based Lee's Pharm.

PTSM: Pharmaceutical Technology Sourcing and Management
Cytovance Biologics anticipates continued expansion plans following acquisition by Hepalink USA

PTSM: Pharmaceutical Technology Sourcing and Management
Manufacturers seek gradual rollout of more targeted FDA quality metrics program.

PTSM: Pharmaceutical Technology Sourcing and Management
Mylan received a warning letter citing violations at its Agila Specialty Formulation Facility, Sterile Products Division, and Onco Therapies Limited sites in India.

PTSM: Pharmaceutical Technology Sourcing and Management
Kate Kuhrt, senior director, Generics and Biotech, Thomson Reuters, spoke with Pharmaceutical Technology Europe about sourcing trends, supply chain challenges, the emerging market outlook, and how it affects European pharmaceutical manufacturers.

PTSM: Pharmaceutical Technology Sourcing and Management
With this acquisition, Source BioScience is now able to offer integrated solutions to customers, with environmentally controlled storage in combination with the required up- or downstream analytical testing services.

PTSM: Pharmaceutical Technology Sourcing and Management
Companies in the US will now have the opportunity to tap into Hermes Pharma’s technical expertise in effervescent and chewable tablets, instant drinks, lozenges, and orally disintegrating granules (ODGs).

PTSM: Pharmaceutical Technology Sourcing and Management
CMC Biologics will manufacture monoclonal antibodies (mAbs) and provide process development services for the PATH Malaria Vaccine Initiative.

PTSM: Pharmaceutical Technology Sourcing and Management
Catalent selects advisory board members to complement Redwood Bioscience Scientific Advisory Board.

PTSM: Pharmaceutical Technology Sourcing and Management
Aprecia Pharmaceutical’s SPRITAM levetiracetam gains FDA approval for the treatment of epilepsy.

PTSM: Pharmaceutical Technology Sourcing and Management
FDA issues draft guidance on dissolution testing for immediate-release solid oral dosage forms.

PTSM: Pharmaceutical Technology Sourcing and Management
Potential for improved product quality and cost/time savings is reviving interest in perfusion technology.
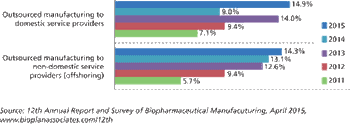
PTSM: Pharmaceutical Technology Sourcing and Management
More contract service options encourage biopharma companies to cut costs by cutting jobs.